All published articles of this journal are available on ScienceDirect.
An Efficient in vitro Assay to Predict Resistance and Pathogenicity in the Fusarium Head Blight-Hordeum Vulgare Pathosystem
Abstract
Background:
Barley (Hordeum vulgare L.) worldwide is affected seriously by Fusarium Head Blight (FHB) disease caused by several Fusarium species. In vitro assays permitting for easy, efficient and reliable prediction of the head blight reaction in the whole plant should be investigated.
Objective and Methods:
The in vitro ability of 16 fungal isolates of four FHB species to confer disease on individual plant organs was evaluated using a coleoptile infection assay. Four quantitative components (Seed Germination (SG), Coleoptile Length (CL), Coleoptile Weight (CW) and Root Weight (RW)) were analyzed in two widely cultivated barley cultivars, Arabi Aswad (AS) and Arabi Abiad (AB), with known quantitative resistance.
Results:
Differences in inoculated pathogenicity and resistance treatments were observed on young plant parts relative to water controls, indicating that these FHB species were found to be suitable for the differential expression of all tested quantitative components. There was a wide variation in pathogenicity among the 16 FHB isolates and susceptibility among AS and AB. The 16 FHB isolates can be separated into the first group with larger number of isolates, upon infection with which AB really was more susceptible to FHB infection than AS, and isolates of the second group with leaser number of isolates for which AS and AB react was the opposite. On AB, rather susceptible, inoculation with FHB species resulted in significantly less SG, CL, CW and RW, compared with AS, which showed a greater resistance. The very good resistance of AS was confirmed by the measurements of quantitative resistance components described in this study. When infected with FHB isolates, all indicators of a more susceptible cultivar seemed to be 10-20% less than those of a resistant cultivar. Moreover, the values of all analyzed components were significantly correlated with the data of pathogenic indices generated in vitro, and under controlled and field conditions with a large diversity depending on AS and AB.
Conclusion:
Appropriate in vitro conditions were determined for the coleoptile infection assay to maximize differences in disease reactions components among FHB isolates and the two barley cultivars. Results suggest that all measured components predict resistance and pathogenicity occurring at the earliest and latest barley development stages during FHB infection. Our data also highlighted, for the first time, the utility of CW and RW for the determination of resistance and pathogenicity in the FHB-barley pathosystem. The coleoptile infection test was confirmed to be adequate to in vitro, growth chamber and field data by the presence of the first group, which prevailed in all other tests generated under different experimental conditions. The in vitro coleoptile infection assay may offer a real possibility of simple, rapid and reliable screening of resistance in barley cultivars and pathogenicity of FHB species.
1. INTRODUCTION
Globally, barley (Hordeum vulgare L.) is the fourth most-produced cereal crop in temperate climate regions. Around 140 million tones per year are produced worldwide, which are mostly utilized as feed (70%) and for beer production (27%) [1]. Barley was originated and domesticated from H. spontaneum prior to 7000 B.C. in the Fertile Crescent encompassing parts of seven countries, including Syria [2, 3]. Barley is still one of the main Syrian cereal crops with a cultivated surface of one million hectares, with more than one million tones in 2011 [4]. Barley production in Syria is entirely based on two old cultivars: Arabi Aswad (black seeded, AS) used mainly for livestock feed and Arabi Abiad (white seeded, AB) for malting and brewing industry. Moreover, these cereal plants have been widely cultivated in Iraq, Jordan and Lebanon [3]. Therefore, the researches on this important group of genetic resources are required for the improvement of commercially valuable traits in barley breeding programs because AS and AB with highest agronomic characteristics include acceptable levels of resistance to abiotic and biotic constraints [2, 3].
Barley, along with other small-grain cereals, can be heavily damaged by pathogenic Fusarium fungi responsible for Fusarium Head Blight (FHB) in all cereal-growing areas of the world. During warm and wet conditions, the disease causes bleaching of the florets resulting in sterility or production of deformed, shrunken, pale and discoloured kernels (tan, orange, brown, pink or red) scattered throughout the head. The result is a reduction in grain yield and quality to the requirements of the malting and brewing industry [5, 6]. The pathogen complex involves diverse Fusarium species, varying in the prevalence of species and mycotoxin spectra depending on geographical factors. F. graminearum and F. culmorum are known to be major FHB species damaging barley. In addition, other FHB causative agents are isolated frequently from infected heads [7-9].
It has been noted that pathogenicity of FHB isolates recovered from various regions within a country and even within species from individual fields are highly variable, and therefore, FHB resistant barley cultivars that are resistant in one location might not exhibit stable results in other locations [9-11]. No complete resistance in barley to FHB was detected, and two primary types of polygenic resistance to disease infection were reported [12]: Type 1 (resistance to initial penetration of the pathogen) and Type II (resistance to spreading within a spike), with type 1 as the predominant type since barley plants exhibit a natural level of type II resistance [13]. The performance of a resistant cultivar primary depends on the broad range of Fusarium species associated, climatic conditions and the interaction between these two variables in a specific location [14]. Therefore, the quest for breeding commercial barley cultivars with desirable agronomic traits and durable resistance continues for the highly variable species of FHB pathogens present in many growing countries [9-14].
A key question in breeding efforts is the screening of barley plants for resistance to various FHB species to distinguish cultivars that are likely to resist variable pathogen populations. Growth chamber and field screening have been a routine procedure associating with various impediments for evaluating barley cultivars for resistance to local FHB populations. Moreover, varying uncontrollable environmental conditions such as temperature, humidity, and the simultaneous presence of other pathogens in field make the interpretation of screening data difficult. In addition, in infected barley heads, symptoms are not distinguishing, can be hidden, or may be confused with other fungal diseases compared with infected wheat heads [10, 15]. The in vitro assays overcame limitations posed by field and growth chamber screening has a great potential to facilitate the analysis of multiple pathogenic isolates and to identify head blight-resistant sources; however, some studies referenced herein did not investigate correlations with the head blight reaction in whole plant [16-20].
Till now, FHB fungi have not been isolated from Syrian barley fields. However, a similar pathogenic range was reported for different fungal isolates recovered from diseased wheat heads on both: AS and durum wheat plants in vitro [21]. Recently, Sakr [22, 23] reported that area under disease progress curve and latent period, out of nine tested components delivered from three in vitro assays in wheat [24], differentiated FHB isolates and barley cultivars, AS and AB. Furthermore, these components were indicators of pathogenicity and quantitative resistance occurring in the whole plant during FHB infection [23, unpublished data]. Sakr [22, 23] recommended to explore other quantitative resistance components in FHB-wheat researches to know whether these components are efficient tools to predict resistance and pathogenicity in an FHB-H. vulgare pathosystem. Soresi et al. [25] found that the coleoptile infection method and head blight tests correlated for durum wheat resistance scores against F. graminearum and therefore, resistance detected in vitro could be predicted for head blight development via coleoptile infection tests. Despite the importance of this assay, there are no associated reports on barley.
An important challenge is to analyze more effective and accurate in vitro disease evaluation methods for detecting pathogen and cultivar differences and investigating if pathogenicity and resistance observed in individual plant organs and earlier growth stages may relate to disease expression in the whole plant. In this context, the goals of the present research were, (1) to clarify the utility of the in vitro coleoptile infection assay for identification FHB resistant cultivars in most planted Syrian barley and pathogenicity of a set of Fusarium fungi, and (2) to examine the relationships between the current data and the findings previously generated using in vitro detached leaf and Petri-dish tests and artificial inoculations under controlled and field conditions for potential prediction of the head blight reaction at seedling and whole plant stages.
2. MATERIALS AND METHODS
2.1. Fungal Isolates and Inoculum Preparation
Sixteen fungal isolates representing four Fusarium species (F. culmorum (F1, F2, F3, F28 and F30), F. verticillioides (synonym F. moniliforme) (F15, F16, F21 and F27), F. solani (F7, F20, F26, F29, F31 and F35), and F. equiseti (F43)) were obtained from heads displaying observable disease symptoms collected during the 2015 growing season in several localities of the Ghab Plain, one of the principal Syrian wheat production areas. All isolates have been morphologically identified on the basis of macroscopic features such as pigmentations and growth rates over the surface of Potato Dextrose Agar (PDA, HiMedia, HiMedia Laboratories) in 9-cm Petri dishes, as well as their microscopic characteristics involving the size of macroconidia, presence of microconidia and chlamydospores [26]. The 16 fungal isolates were also molecularly identified [Sakr, unpublished data]. To ensure adequate pathogenicity on the tested barely plants, pathogenic reactions were analyzed with the main Fusarium species present in Syria because FHB pathogens were not recovered from Syrian barley fields till now. For long term storage, fungal cultures were preserved in sterile distilled water at 4 °C and freezing at -16 °C [27].
For inoculum preparation, the isolates were placed on PDA Petri dishes and incubated in an incubator (JSPC, JS Research Inc) for ten days at 22ºC under continuous darkness to allow mycelial growth and sporulation. Following growth, 10 ml of sterile distilled water were added to each dish, and the resulting spore suspensions were adjusted to 2 × 105 spores/ml for inoculation following a count in a hemacytometer.
2.2. Barley Cultivars
Pathogenicity and quantitative resistance evaluations were performed using two barley cultivars: Arabi Aswad (AS) and Arabi Abiad (AB). AS and AB were selected because they are currently the most important barley cultivars in Syria. AS is adapted to drier areas and popular in the northeast Syria. AB is adapted and primarily planted in the wetter areas in western and northwestern Syria. Both genetically different cultivars are two-rowed, with thin stems and high tillering ability [2, 3]. AB is more susceptible to FHB infection than AS in the resistance as measured by the Latent Period (LP) of detached leaf inoculation and standardized Area Under Disease Progress Curve (AUDPCstandard) of Petri-dish inoculation detected in vitro [22] and in the adult FHB resistance type I under controlled and field conditions during the two growing seasons 2017/18 and 2018/19 [Sakr, unpublished data]. Furthermore, pathogenic reactions of the 16 tested FHB isolates in these two cultivars were determined using in vitro LP and AUDPCstandard methodologies [23] and disease incidence detected using an artificial head inoculation generated under controlled and field conditions [Sakr unpublished data] (Table 1). Therefore, we were able to examine the relationships between the current findings with the previous results of in vitro and spraying inoculations in the growth chamber and field.
2.3. Quantitative Component Tests in vitro
The ability of 16 fungal isolates of four FHB species to confer disease on young plant organs in vitro was evaluated using a coleoptile infection assay, according to Soresi et al. [25].
A Petri dish with 15 barley seeds was imbibed in 4 mL of 16 FHB isolate suspensions (2 × 105 spores/ml), or in sterile distilled water for the control treatment. After 15 min, the excess suspension was decanted and the inoculated barley seeds were planted on filter paper (Filtrak, Thermo Fisher Scientific Inc.) placed on 0.5% agar in Petri dishes, then were incubated at 15ºC with 16 h photoperiod and were arranged in a complete randomized design with three replicates. Three Petri-dishes per replicate were left non-inoculated as a control treatment. Six days after inoculation, four components were recorded: seed germination, coleoptile length, coleoptile weight and root weight. Germinated seeds were calculated in each inoculated 15-seed dish and expressed as a percentage of that of the non-inoculated dish mean. Roots and coleoptiles were harvested from each seedling and their lengths were measured using calipers and weights were determined using an analytical balance. Coleoptile and root measures were registered in each germinated individual dish and demonstrated as a percentage of the control dish mean. The experiment was repeated. Results were similar between the two in vitro coleoptile infection experiments and the data from the second experiment are presented.
The two components analyzed herein: seed germination and coleoptile length were analyzed in the past with the in vitro Petri-dish test on barley seeds [22, 23]. However, different inoculum concentration, infection methods and growth conditions were assessed for the in vitro Petri-dish test as compared with the coleoptile infection assay. In the Petri-dish inoculation assay [28], a 6 ml suspension of conidia at 1×106 spores/ml was used and the seeds were submerged in the fungal inoculum suspension and then were placed into a Petri-dish with sterile double-layer filter paper. Infected treatments were incubated at 22oC in the dark in the Petri-dish inoculation assay.
2.4. Quantitative Component Tests Under Controlled And Field Conditions
All the 16 FHB isolates assayed by coleoptile infection assay were previously tested in a growth chamber (at 20°C day/night temperature, and 16/8 h light/dark) and in the field at the Deir Al-Hajar Agricultural Experiment Station, located south east of Damascus, Syria (33°20′ N, 36°26′ E) at 617 m above sea level during the two growing seasons 2017/18 and 2018/19 on AS and AB to assess resistance and pathogenicity [Sakr, unpublished data]. In brief, when the spikes reached 50% anthesis, the experimental plants were spray-inoculated with 5 × 104 spores/ml of each of the 16 FHB isolates. Control plants were sprayed with sterile distilled water. Inoculated spikes were covered with polyethylene bags for 48 h (100% relative humidity) to promote infection. FHB incidence (% of symptomatic spikes) was estimated 21 days after inoculation as the percentage of spikes in a plant with visible FHB symptoms.
2.5. Statistical Analyses
Data were subjected to Analysis of Variance (ANOVA) using the DSAASTAT add-in version 2011. Before statistical analysis, the percentages of all quantitative components were transformed using the angular transformation to stabilize variances. Tukey's test was used to compare the means at a significant level of 5%. The sample correlation coefficients (Pearson r) were calculated using overall values per isolates at a significant level of 5%.
3. RESULTS
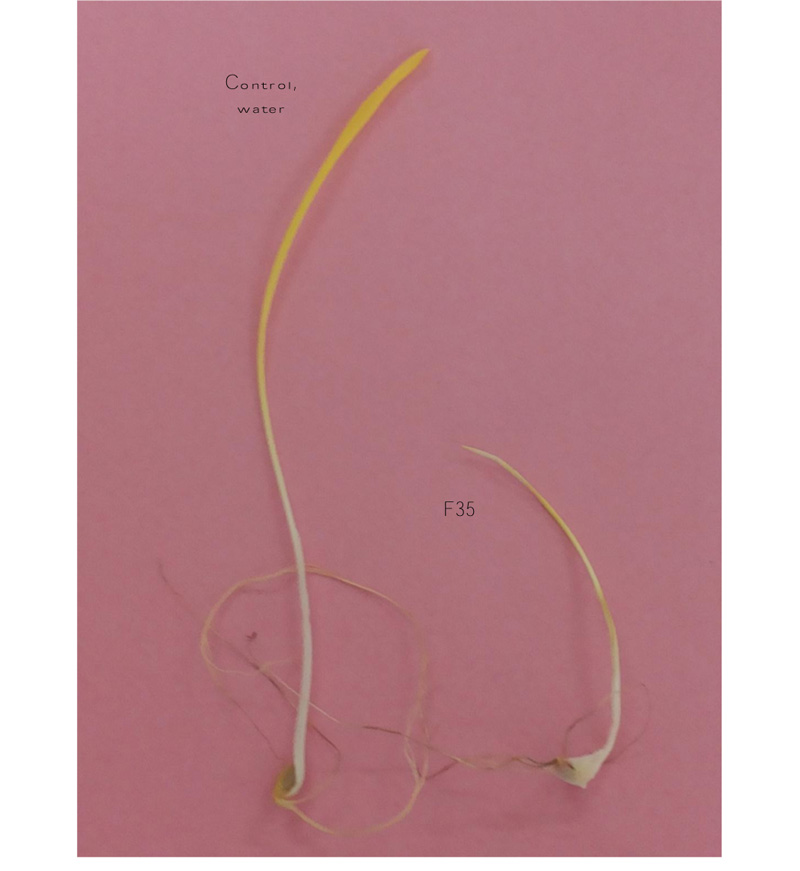
In general, the exposure of inoculated pathogenicity and resistance treatments for the four quantitative components on AS and AB to a set of 16 Fusarium isolates reduced mean values compared to the non-inoculated control, revealing an efficient influence of the four FHB species on the growth of these two cultivars. FHB infected seedlings were identified by mycelia covering the seed surfaces and/or reddish discoloration on the coleoptiles and roots, whereas the water plants did not show any disease symptoms (Fig. 1).
F-test values from analyses of variance for Seed Germination (SG), Coleoptile Length (CL), Coleoptile Weight (CW) and Root Weight (RW) were summarized in Table 2 and revealed statistically significant differences in pathogenicity among the 16 FHB isolates and susceptibility among AS and AB. Scores (% of control) for SG, CL, CW and RW are presented in Table 3. No significant correlation was detected between the values of GS, CL, CW and RW showed in Table 3 for AS and AB (r=0.021 ns, r=0.293 ns, r=-0.051 ns and r=0.218 ns, respectively).
For all components studied, there was a significant interaction between the 2 factors: FHB isolate and barley cultivar. There were significant correlations between the four quantitative components (SG, CL, CW and RW) obtained with the in vitro coleoptile infection assay (Table 4).
When a comparison of 16 fungal isolates among themselves for each barley cultivar was conducted in this study, fungal isolates with lower values of SG, CL, CW and RW were considered as more pathogenic isolates. When a comparison of two barley cultivars among themselves for each isolate was conducted in this study, a barley cultivar with higher values of SG, CL, CW and RW was considered as resistant.
The values of reductions of SG, CL, CW and RW were calculated in the present research as % of the average of % for different isolates on one grade from the average of % of another cultivar.
3.1. Seed Germination (SG)
The number of germinated barley seeds decreased in treatments inoculated with 16 FHB isolates. On AS, the values for SG ranged from ~ 53% for the most pathogenic isolates F7 and F35 (F. solani) and F43 (F. equiesti) to 79% for the least pathogenic isolate F1 (F. culmorum). On AB, the values for SG ranged from 29% for the most pathogenic isolate F30 (F. culmorum) to 71% for the least pathogenic isolate F35 (F. solani). However, there were substantial differences in GS on AS and AB, with reductions ranging from 21% to 48% for AS and from 29% to 71% for AB relative to non-inoculated controls. Although both barley cultivars were not differently affected by all tested isolates except for F3, F28 and F30 (F. culmorum), F29 (F. solani), and F16 and F21 (F. verticillioides); AS seemed to exhibit more GS scores than AB. Thus, AS appeared to be more resistant as measured by SG than AB. Consequently, reduced SG of AB seemed to be 15.6% less than AS.
3.2. Coleoptile Length (CL)
The length of diseased coleoptiles was less than that of the healthy coleoptiles that reached 10.2 mm and 10.3 mm for AB and AS, respectively, regardless of the FHB isolate. On AS, the values for CL ranged from 58% for the most pathogenic isolate F35 (F. solani) to 81% for the least pathogenic isolate F1 (F. culmorum). On AB, the values for CL ranged from ~ 40% for the most pathogenic isolates F3, F28 and F30 (F. culmorum) and F7 and F29 (F. solani) to 81% for the least pathogenic isolate F27 (F. verticillioides). However, the reductions in coleoptile growth also ranged widely on AS and AB, ranging from 19% to 42% for AS and from 19% to 67% for AB. Although the both barley cultivars were not differently affected by all tested isolates except for F3, F28 and F2 (F. culmorum) and F7 (F. solani); AS seemed to exhibit more CL scores than AB. Thus, AS appeared to be more resistant as measured by CL than AB. Consequently, the reduced CL of AB seemed to be 11.9% less than AS.
3.3. Coleoptile Weight (CW)
The weight of diseased coleoptiles was less than that of the healthy coleoptiles that reached 0.97g and 0.85g for AB and AS, respectively, irrespective of the FHB isolate. On AS, the values for CW ranged from 40% for the most pathogenic isolate F43 (F. equiesti) to 82% for the least pathogenic isolate F15 (F. verticillioides). On AB, the values for CW ranged from ~ 30% for the most pathogenic isolates F3, F28 and F30 (F. culmorum) and F7 (F. solani) to 73% for the least pathogenic isolate F27 (F. verticillioides). However, intrinsic differences in CW were detected on AS and AB, with reductions ranging from 18% to 60% for AS and from 27% to 71% for AB relative to non-inoculated controls. Although both barley cultivars were not differently affected by all tested isolates except for F3, F28 and F2 (F. culmorum), F7 and F29 (F. solani), F15 (F. verticillioides) and F43 (F. equiesti); AS seemed to exhibit more CW scores than AB. Thus, AS appeared to be more resistant as measured by CW than AB. Consequently, reduced CW of AB seemed to be 18.5% less than AS.
|
Fungal Isolates (Identification) |
LP | AUDPCstandard | DI % (CC) | DI % (FC,17/18) | DI % (FC, 18/19) | |||||
| AS | AB | AS | AB | AS | AB | AS | AB | AS | AB | |
| F1(F. culmorum ) | 7.7 ef A |
8.1 f A |
0.22 f A |
0.35 ef B |
24 fg A |
42 de B |
31 efgh A |
39 ef A |
30 efg a |
42 cd A |
| F2(F. culmorum) | 5.8 bc A |
3.6 ab B |
0.29 def A |
0.26 gh A |
26 efg A |
33 ef A |
26 gh A |
31 fg A |
23 g A |
33 de A |
| F3(F. culmorum) | 4.4 a A |
4.9 cd A |
0.39 abc A |
0.58 c B |
35 bcdef A |
64 bc A |
31 efgh A |
69 b B |
32 efg A |
65 b B |
| F28(F. culmorum) | 5.8 bc A |
6.3 e A |
0.29 def A |
0.45 d B |
32 cdef A |
40 e A |
32 defgh A |
49 de B |
36 de A |
46 c A |
| F30(F. culmorum) | 7.5 ef A |
8.4 fg A |
0.34 bcd A |
0.70 a B |
31 defg A |
85 a B |
44 bc A |
62 bc B |
46 bc A |
60 b B |
| F7(F. solani) | 9.0 g A |
9.4 g A |
0.45 a A |
0.67 ab B |
40 abcd A |
61 bc B |
58 a A |
67 b A |
61 a A |
65 b A |
| F20(F. solani) | 8.0 fg B |
5.6 de A |
0.40 ab A |
0.40 de A |
32 cdef A |
60 bc B |
44 bcd A |
60 bcd B |
46 bc A |
55 b A |
| F26(F. solani) | 7.9 fg B |
5.6 de A |
0.39 abc A |
0.40 de A |
24 fg A |
52 cd B |
43 bcd A |
52 cd A |
42 bcd A |
55 c B |
| F29(F. solani) | 7.5 ef A |
8.4 fg A |
0.38 abc A |
0.60 bc B |
45 ab A |
66 b B |
42 bcde A |
84 a B |
39 cde A |
78 a B |
| F31(F. solani) | 6.5 cde B |
4.2 abc A |
0.33 bcde A |
0.30 fgh A |
42 abc B |
27 fg A |
39 cdef B |
27 g A |
42 bcd A |
32 e A |
| F35(F. solani) | 7.7 ef A |
5.3 cde B |
0.39 abc A |
0.38 def A |
43 abc A |
34 ef A |
46 abc B |
30 fg A |
47 bc B |
32 e A |
| F15(F. verticillioides) | 4.4 a A |
3.5 ab A |
0.22 f A |
0.25 h A |
20 g A |
27 fg A |
24 h A |
27 fg A |
26 fg A |
31 e A |
| F16(F. verticillioides) | 5.0 ab A |
3.1 a B |
0.31 cde A |
0.41 de A |
38 bcde A |
37 ef A |
28 fgh A |
54 cd B |
31 efg A |
49 c B |
| F21(F. verticillioides) | 7.1 def A |
5.4 cde B |
0.35 bcd A |
0.38 de A |
32 cdef A |
35 ef A |
35 cdefgh A |
50 cde B |
31 efg A |
45 c B |
| F27(F. verticillioides) | 6.3 cd A |
5.8 de A |
0.25 ef A |
0.22 h A |
37 bcde B |
18 g A |
37 cdefg B |
25 g A |
35 def A |
33 de A |
| F43(F. equiesti) | 8.0 fg A |
4.7 bcd B |
0.40 ab A |
0.33 efg A |
52 a B |
20 g A |
52 ab B |
37 fg A |
49 b A |
40 cde A |
| P (F) isolates= 2.4E-14 |
P (F) isolates= 6.24E-14 |
P (F) isolates= 5.89E-13 |
P (F) isolates= 0.990164 |
P (F) isolates= 1.22E-12 |
||||||
| P (F) cultivars= 7.38E-06 |
P (F) cultivars= 1.13E-07 |
P (F) cultivars= 2.03E-08 |
P (F) cultivars= 0.776472 |
P (F) cultivars= 1.15E-07 |
||||||
| P (F) interactions= 0.000977 |
P (F) interactions= 2.37E-06 |
P (F) interactions= 1.15E-14 |
P (F) interactions= 0.803845 |
P (F) interactions= 2.24E-07 |
||||||
| Source of Variation | df | SG | CL | CW | RW |
| Isolate (I) | 15 | 7.92E-05 | 0.00016 | 0.000437 | 1.24E-05 |
| Cultivar (C) | 1 | 4.34E-05 | 0.001254 | 3.2E-06 | 0.001473 |
| I × C | 15 | 0.000121 | 0.010579 | 0.000152 | 0.001324 |
| Error | 64 | ||||
| CV (%) | 19.3 | 19.2 | 19.5 | 19.4 |
SG = seed germination; CL = coleoptile length; CW = coleoptile weight; RW = root weight.
|
Fungal Isolates (Identification) |
SG (% of Control) | CL (% of Control) | CW (% of Control) | RW (% of Control) | ||||
| AS | AB | AS | AB | AS | AB | AS | AB | |
| F1(F. culmorum ) | 79 e A | 63 de A | 81 c A | 64 bcd A | 71 cd A | 61 cde A | 75 de A | 55 bc B |
| F2(F. culmorum) | 72 cde A | 65 de A | 69 abc A | 75 cde A | 72 cd A | 71 de A | 65 bcd A | 71 def A |
| F3(F. culmorum) | 68 bcde A | 43 bc B | 65 ab A | 40 a B | 75 cd A | 35 a B | 61 bcd A | 36 a B |
| F28(F. culmorum) | 70 bcde A | 36 ab B | 74 bc A | 40 a B | 68 cd A | 36 a B | 65 bcd A | 41 ab B |
| F30(F. culmorum) | 60 abc A | 29 a B | 64 ab A | 33 a B | 68 cd A | 29 a B | 68 cd A | 39 a B |
| F7(F. solani) | 53 a A | 42 abc A | 60 ab A | 36 a B | 52 ab A | 32 a B | 52 ab A | 41 ab A |
| F20(F. solani) | 58 ab A | 63 de A | 65 ab A | 62 bc A | 68 cd A | 58 cd A | 59 ab A | 65 cde A |
| F26(F. solani) | 65 abcd A | 58 de A | 62 ab A | 62 bc A | 66 bc A | 57 cd A | 63 bcd A | 68 cdef A |
| F29(F. solani) | 58 ab A | 35 ab B | 63 ab A | 42 a B | 64 bc A | 38 ab B | 53 ab A | 35 a B |
| F31(F. solani) | 60 abc A | 53 cd A | 65 ab A | 60 b A | 62 bc A | 52 bc A | 71 cde A | 63 cde A |
| F35(F. solani) | 54 a A | 71 e A | 58 a A | 65 bcd A | 52 ab A | 61 cde A | 62 bcd A | 55 bc A |
| F15(F. verticillioides) | 76 de A | 65 de A | 81 c A | 78 de A | 82 d A | 63 cde B | 85 e A | 82 f A |
| F16(F. verticillioides) | 75 de A | 52 cd B | 71 abc A | 63 bc A | 71 cd A | 53 c A | 71 cde A | 40 a B |
| F21(F. verticillioides) | 63 abcd A | 34 ab B | 60 ab A | 65 bcd A | 62 bc A | 61 cde A | 65 bcd A | 60 cd A |
| F27(F. verticillioides) | 59 abc B | 85 f A | 69 abc A | 81 e A | 61 bc A | 73 e A | 51 ab B | 75 ef A |
| F43(F. equiesti) | 52 a A | 65 de A | 63 ab A | 71 bcde A | 40 a B | 63 cde A | 45 a B | 60 cd A |
| - | SG | CL | CW | RW |
| SG | 1.000 | - | - | - |
| CL | 0.898*** | 1.000 | - | - |
| CW | 0.833*** | 0.886*** | 1.000 | - |
| RW | 0.685** | 0.800*** | 0.835*** | 1.000 |
3.4. Root Weight (RW)
The weight of diseased roots was less than that of the healthy coleoptiles that reached 0.58g and 0.49g for AB and AS, respectively, regardless of the FHB isolate. On AS, the values for RW ranged from 45% for the most pathogenic isolate F43 (F. equiesti) to 85% for the least pathogenic isolate F15 (F. verticillioides). On AB, the values for RW ranged from ~ 38% for the most pathogenic isolates F3 and F30 (F. culmorum), F29 (F. solani) and F16 (F. verticillioides) to 82% for the least pathogenic isolate F15 (F. verticillioides). However, the reductions in coleoptile growth varied widely on AS and AB, ranging from 15% to 55% for AS and from 18% to 65% for AB. Although both barley cultivars were differently affected by all tested isolates except for F2 (F. culmorum), F7, F20, F26, F31 and F35 (F. solani), and F16 and F21 (F. verticillioides); AS seemed to exhibit more RW scores than AB. Thus, AS appeared to be more resistant as measured by RW than AB. Consequently, reduced RW of AB seemed to be 12.7% less than AS.
3.5. Existence of Two Fungal Groups Differed in Pathogenicity With Respect To AS and AB For All Pathogenic Components Generated Under Several Experimental Conditions
The 16 FHB isolates can be divided with statistically significant differences in pathogenicity with respect to the two tested cultivars into the first group, upon infection with which AB was more susceptible to FHB infection than AS and isolates of the second group for which AS and AB react was the opposite. The remaining isolates gave an intermediate reaction and can be assigned to the first or second group (Tables 1 and 3). For SG, 12 isolates i.e, F1, F2, F3, F28, F30, F7, F26, F29, F31, F15, F16 and F21can be separated into the first group and 4 isolates i.e, F20, F35, F27 and F43 can be divided into the second group. For CL, 11 isolates i.e, F1, F3, F28, F30, F7, F20, F26, F29, F31, F15 and F16 can be separated into the first group and 5 isolates i.e, F2, F35, F21, F27 and F43 can be divided into the second group. For CW, 13 isolates i.e, F1, F2, F3, F28, F30, F7, F20, F26, F29, F31, F15, F16 and F21 can be separated into the first group and 3 isolates i.e, F35, F27 and F43 can be divided into the second group. For RW, 11 isolates i.e, F1, F3, F28, F30, F7, F29, F31, F35, F15, F16 and F21 can be separated into the first group and 5 isolates i.e, F2, F20, F26, F27 and F43 can be divided into the second group. For LP, 10 isolates i.e, F2, F20, F26, F31, F35, F15, F16, F21, F27 and F43 can be separated into the first group and 6 isolates i.e, F1, F3, F28, F30, F7 and F29 can be divided into the second group. For AUDPCstandard, 11 isolates i.e, F1, F3, F28, F30, F7, F20, F26, F29, F15, F16 and F21 can be separated into the first group and 5 isolates i.e, F2, F35, F15, F27 and F43 can be divided into the second group. For disease incidence measured under controlled conditions, 11 isolates i.e, F1, F2, F3, F28, F30, F7, F20, F26, F29, F15 and F21 can be separated into the first group and 5 isolates i.e, F31, F35, F16, F27 and F43 can be divided into the second group. For disease incidence measured under field conditions during the growing season 2017/18, 12 isolates, i.e., F1, F2, F3, F28, F30, F7, F20, F26, F29, F15, F16 and F21 can be separated into the first group and 4 isolates, i.e., F31, F35, F27 and F43 can be divided into the second group. For disease incidence measured under field conditions during the growing season 2018/19, 12 isolates, i.e., F1, F2, F3, F28, F30, F7, F20, F26, F29, F15, F16 and F21 can be separated into the first group and 4 isolates, i.e., F31, F35, F27 and F43 can be divided into the second group.
3.6. Comparison Among Values of Differences in Resistance Between AS and AB Under Several Experimental Conditions
When infected with FHB isolates, all indicators of a more susceptible cultivar, AB, seemed to be 10-20% less than those of a resistant cultivar, AS. Thus, these values of differences in resistance between AS and AB are comparable with those obtained under several experimental conditions, the latent period of AB was 14.7% less than AS, AUDPC standard of AS was less 19.0% than AB [22], disease incidence for Type I resistance under controlled conditions of AS was 22.7% less than AB, disease incidence for Type I resistance under field conditions during the 2017/18 growing season of AS was 20.8% less than AB and disease incidence for Type I resistance under field conditions during the 2018/19 growing season of AS was 18.8% less than AB [Sakr, unpublished data].
3.7. Correlations Between Pathogenicity Components Generated Under Several Experimental Conditions
The values of SG, CL, CW and RW were significantly correlated with previously obtained values of in vitro LP and AUDPCstandard and disease incidence generated under controlled and field conditions during the two growing seasons 2017/18 and 2018/19 on AS and AB (Table 5).
Table 5.
| Pathogenicity Components | LP | AUDPCstandard | DS % (CC) | DS%(FC,17/18) | DS%(FC, 18/19) | |||||
| AS | AB | AS | AB | AS | AB | AS | AB | AS | AB | |
| SG | -0.655** | -0.531* | -0.732** | -0.775*** | -0.729** | -0.659** | -0.888*** | -0.727** | -0.823*** | -0.673** |
| CL | -0.528* | -0.653** | -0.871*** | -0.935*** | -0.570* | -0.816*** | -0.717** | -0.787*** | -0.635** | -0.783*** |
| CW | -0.676** | -0.578* | -0.569* | -0.916*** | -0.785*** | -0.777*** | -0.815*** | -0.758*** | -0.720** | -0.762*** |
| RW | -0.508* | -0.505* | -0.628** | -0.843*** | -0.702** | -0.642** | -0.688** | -0.729** | -0.571* | -0.761*** |
4. DISCUSSION
To our best knowledge, this in vitro research set out for the first time to predict the resistance of two morphologically, physiologically and genetically different barley cultivars [2, 3] and pathogenicity in a collection of four Syrian Fusarium species: F. culmorum, F. verticillioides, F. solani and F. equiseti in the whole plant. The coleoptile infection method adopted for in vitro growth measurements herein has been used previously for Fusarium fungi on durum wheat plants [25]. Although it is likely that there are more resistance and pathogenicity components than reported to date, our data revealed that this test may facilitate progress in the study of plant-Fusarium interactions and in the resistance evaluation in breeding programs with the ultimate aim of disease control in the field.
The fact that resistance and pathogenicity detected in the in vitro coleoptile infection assay was of greater importance in the head infection conducted under controlled and field conditions is consistent with the current understanding of the infection process. FHB pathogens cannot penetrate the thick-walled epidermal cells on the exterior surface of the glume, lemma and palea [12]. However, hyphal development and growth on the outside of the glume allow the fungi entry by several different pathways, which is an important first step allowing it to reach more susceptible sites within the glumes or floret [12], where grain development occurs. Thus, disease development is manifested through the appearance of symptoms such as mycelia covering the seed surfaces and/or reddish discoloration on the affected plant part.
Appropriate in vitro conditions were determined for the coleoptile infection assay to maximize differences in disease reaction components among FHB species and the two barley cultivars. During our research, differences in inoculated treatments were observed on young plant parts relative to water controls, indicating that the FHB species used in this experiment were found to be suitable for the differential expression of all tested quantitative components. Compared with previous SG and CL analyses in a Petri-dish inoculation assay, which showed no significant differences among the same fungal isolates and barley cultivars [22, 23], it seems that inoculum concentration, infection methods and growth conditions permitted to assess substantial differences in SG and CL. Different infection methods could help to identify the level of resistance/susceptibility of wheat varieties under controlled and field conditions [29].
According to Soresi et al. [25], quantitative resistant wheat cultivars are identified by high values of SG, CL, CW and RW compared with the susceptible one. In the current experiment, the differences in resistance/susceptibility levels between AS and AB were recognized for the four tested components. It seems that these parameters measured in work are indictors of mechanisms of resistance occurring in the whole plant during FHB infection. On AB, rather susceptible under several experimental conditions, inoculation with FHB species resulted in significantly less SG, CL, CW and RW, compared with AS, which showed a greater resistance. The very good resistance of AS was confirmed by the measurements of quantitative resistance components described in this study. When infected with Fusarium pathogens, all indicators of a more susceptible cultivar, AB, appear to be 10-20% less than those of a resistant cultivar, AS. As expected, these results confirmed previous in vitro and controlled and field findings that AB was more susceptible to FHB infection than AS [Sakr [22], unpublished data], suggesting that the assessment of resistance level is repeatable and stable under several experimental conditions.
The four components evaluated were informative for FHB prediction at the earliest and latest barley development stages. The variation in the relative strength of relationships between resistance as measured by SG, CL, CW and RW and the resistance as measured by LP and AUDPCstandard detected in vitro [22] and the adult FHB resistance type I under controlled and filed conditions [Sakr, unpublished data] indicate that the measured differences in the in vitro coleoptile infection assay were different aspects of similar response mechanisms against FHB at the seedling and whole plant stages. This also is expected since many additional factors governing resistance in the whole plant are not measured using the in vitro assays [30]. In accordance with our data, Browne [30, 31] reported that higher SG was related to greater FHB type II resistance. Also, Soresi et al. [25] demonstrated that CL was more indicative of FHB type II resistance. However, SG and CL assays are two techniques generally utilized for the estimation of wheat cultivar resistance. In contrast, Shin et al. [32] noted that reductions in SG were poorly correlated with both: type I and type II in adult wheat plants. In addition, the weight of coleoptiles and roots included in our in vitro blight assay did determinate the resistance/susceptibility level in the FHB-barley pathosystem for the first time.
The significance of differences in SG, CL, CW and RW are indications of pathogenicity of individual isolates [33]. SG, CL, CW and RW did distinguish isolates within and among species. The wide range of variability of pathogenicity among FHB isolates in our study has been supported by another study investigating the pathogenicity of several FHB species [9, 11]. Mutation, genetic recombination, or selection in the 16 FHB isolates may play a basic role in pathogenesis [34]. Our data are in accordance with those found by Purahong et al. [28]; they observed highly significant differences in pathogenicity of F. graminearum on wheat as measured by CL. Brennan et al. [35] reported that the reduction of the CL has been related to pathogenicity. Our results did not agree with those reported by Purahong et al. [28]; they observed that reductions in GS were not significant to differentiate fungal isolates. There are some studies that demonstrate the reduction of SG of wheat seeds caused by F. graminearum [7, 18]: so when the barley seeds inoculated with the four tested FHB species in the coleoptile infection assay are infected, their SG rates are decreased compared with the water control. Our data also highlighted, for the first time, the utility of CW and RW for the determination of pathogenicity in FHB-barley pathosystem. In addition, certain reports indicated symptoms on root after F. equiseti (and other soil-born Fusarium species) infection [36]. In fact, retarded or no root development can cause life-threatening constraints for seedlings under water-deficit field environment. Anyway, it could be more important than the inhibition of cotyledon elongation. Furthermore, the four FHB species used in this experiment are known as able to mycotoxin production. Thus, the capability of 16 fungal isolates to cause reddish discoloration on the coleoptiles and roots in varying amounts might be mainly the result of the phytotoxic action of these metabolites [12].
It should be pointed that a complex genotype interaction was found between FHB isolates and barley cultivars for SG, CL, CW and RW revealed by the presence of cultivar-specific pathogenicity, suggesting that pathogenicity mechanisms and resistance genes may be different to disease caused by individual FHB species and the isolate-specific effectiveness may lead to erosion of barley quantitative resistance to FHB invasion. Strong evidence was reported for specific pathogenicity interactions among fungal species implicated in the FHB complex and barley plants [7, 23]. However, further investigation is required in order to draw any final conclusions. The four pathogenicity components involved in this assay were found to be correlated, suggesting that these components are genetically identical, and also reflecting into complex polygenic nature of pathogenicity in the interaction in the FHP-barley system, which are not fully understood [37].
Correlations were obtained between the data of several pathogenicity components and LP, AUDPCstandard, disease incidence previously generated in vitro and in the growth chamber and in the field for AS and AB. When considered together, these independent pathogenic studies indicate the usefulness of SG, CL, CW and RW for FHB evaluation concerning both the pathogen and the host. In parallel, Purahong et al. [28] reported positive relationships of AUDPC estimations and FHB evaluations obtained by spray inoculation of F. graminearum across four durum wheat cultivars in the growth chamber and field. They found high correlations between these three parameters [28], which is similar to our results. Also, a weak and negative correlation of wheat seed germination value caused by Microdochium majus and FHB rating obtained by head inoculation of F. graminearum in the field was observed by Browne [30]. Therefore, the in vitro components, SG, CL, CW and RW, predict pathogenicity occurring at the earliest and latest barley development stages during FHB infection.
It is widely accepted that resistance and pathogenicity are horizontal and non-species specific in an FHB-cereal pathosystem [12]; the absence of isolate × cultivar specific interaction suggests a common infection strategy of FHB isolates and modulates identically by cereal cultivars of contrasted FHB susceptibility [38]. FHB isolates revealed large differences in pathogenicity, which were mostly unchanged when facing hosts of contrasted susceptibility [38]; this implies that the tested isolates should not be divided into groups when interacting with cereals. Although the tested 16 FHB isolates analyzed with the coleoptile infection test were separated into two groups, this test was confirmed to be adequate to in vitro, growth chamber and field data by the presence of the first group, which prevailed in all other tests generated under different experimental conditions. The first group with a larger number of isolates, upon infection with which AB really was more susceptible to FHB infection than AS, and isolates of the second group with leaser number of isolates for which AS and AB react was the opposite. There were significant cultivar × isolate interactions observed in the present study, which agree with previous reports on barley conducted under controlled conditions [9]. The presence of isolates of group 2 indicates that these two barley cultivars may each possess different genes for resistance to the respective FHB species [9]. Since only two barley cultivars were analyzed here, an additional study using a large sample of available Syrian barley cultivars is needed to validate our results in vitro, in the growth chamber and field.
CONCLUSION
During our investigation, the in vitro coleoptile infection assay overcame the limitations associated with time and space dependency of field and growth chamber evaluations allows for the identification of susceptibility levels in AS and AB proposed previously as a promising level of resistance in the barley breeding programs. Furthermore, the four evaluated quantitative components can be used for screening the most pathogenic isolates of different FHB species for FHB resistance breeding of barley. Although significant interaction of isolate by cultivar was detected, the tested method was adequate for other disease reactions under different experimental conditions. The in vitro coleoptile infection assay has a high potential to simplify the advance of research into the barley-FHB pathosystem since it offers a real possibility of simple, rapid and reliable prediction of resistance in barley cultivars and pathogenicity of FHB species. Further research on quantitative trait loci associated with pathogenicity in fungi and resistance in barley plants should be investigated to better understand plant-pathogen interactions on the molecular level generated in the expression of FHB pathogenicity and resistance. Also, inheritance studies to find out the genetics of resistance should be sought.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The author would like to thank the Atomic Energy Commission of Syria for financial support.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
I gratefully thank Dr. I. Mubarak for helping in stats. The unknown Reviewers are thanked for constructive comments on this manuscript.


