All published articles of this journal are available on ScienceDirect.
Biobutanol Production from Plant Biomass
Abstract
Microbiological conversion of biosphere renewable resources to produce useful products, in particular biofuels, is currently one of the pressing problems of biotechnology. To establish a microbiological production of biobutanol at an industrial scale, strains with high-yield solvent production on plant biomass as a cheap substrate are needed.
This paper summarizes the main outcomes of the authors’ original research focused on a) obtaining new butanol-producing strains of Clostridium genus, b) testing different sources of non-food raw material as a substrate for fermentation. A comparison of different methods of biomass pretreatment and their efficiency for the accumulation of butanol in the liquid medium is also reported.
In particular, the efficiency of butanol production by C. acetobutylicum strains isolated or mutagenized by the authors on a) ground green rapeseed, switchgrass, sweet sorghum, soybean, wheat biomass; b) components of switchgrass after thermobaric hydrolysis and c) paper mill sludge from the pulp as substrates is reported. This paper also highlights the progress made concerning substrate pre-treatment and optimization of cultivation conditions to increase butanol production. Finally, future directions to optimize the different biotechnological steps leading to butanol production are discussed.
1. INTRODUCTION
Biobutanol is considered one of the most promising renewable biofuels [1]. Due to its high calorific value and low hydrophilicity, butanol is more like gasoline than ethanol. However, a large scale industrial production of biobutanol is still restrained by low efficiency of its production technology [2]. Butanol is a principal end product of acetone-butanol-ethanol (ABE) fermentation by certain species of anaerobic, spore-forming gram-positive microorganisms. Among them, Clostridium acetobutylicum can mostly produce butanol and acetone.
Biobutanol, as well as bioethanol, can be obtained by both fermentations of traditional sugar- or starch-containing raw materials (1st generation biobutanol) and fermentation of lignocellulosic raw materials from biomass (2nd generation biobutanol). Biobutanol produced in the process of ABE fermentation of biomass by Clostridia spp. has the same characteristics as the butanol obtained by chemical synthesis [3, 4]. The main factor influencing the profitability of the traditional ABE fermentation is the relatively high cost of starch- (corn, wheat, millet, etc.) and sugar- (molasses, sorghum, etc.) containing substrates [5-9]. It is this factor and the ability of saccharolytic Clostridia to use various carbohydrates as substrates that have triggered the research for the production of biobutanol from alternative and renewable raw materials [10, 11].
Earlier works [12-15] on fermentation of various carbohydrates for the production of acetone and butanol demonstrated that some sugars such as glucose, fructose, mannose, sucrose, lactose, starch and dextrose are utilized comprehensively, while galactose, xylose, arabinose, raffinose, melezitose, inulin and mannitol are utilized only partially, and trehalose, rhamnose and melibiose are virtually unutilized. It is known that lignocellulose is a principal structural component of numerous agricultural and wood waste products. A significant amount of residual biomass can potentially be recycled for a variety of products, including biofuel [16]. Bioconversion of lignocellulosic waste for the production of biofuel could make a significant contribution to overcoming numerous environmental and economic problems [17-26].
Over the past two decades, significant progress has been made in the butanol biotechnology, especially in the cultivation process through (1) butanol-titer improvement, (2) by-products isolation or elimination, (3) increased tolerance of bacteria to butanol concentration and (4) utilization of not only pure sugars, but also lignocellulosic biomass hydrolysates as substrates for bacteria. The use of renewable raw materials - lignocellulosic biomass - and the possibility of partial or complete replacement of food raw materials with non-food raw materials is important progress on the way to establishing production of biobutanol at an industrial scale [27-31]. Here we review recent advancements on the use of non-food plant raw materials as a substrate for fermentation and compare effectiveness of different methodologies of green biomass pretreatment for the accumulation of butanol in a liquid medium.
2. SUBSTRATE AND ITS COMPONENTS
Lignocellulose, as the main component of almost all plant biomass, is the most common renewable resource and practically unlimited cultivation substrate for the production of biofuels and other valuable chemicals [11, 14, 16, 17]. Lignocellulose is composed of lignin, hemicellulose and cellulose. The percentage ratios of the lignocellulose components may vary depending on the type of raw material. Each of the lignocellulose components (hemicellulose, cellulose and lignin) can, if properly treated, be used in the production of biofuels.
Hemicelluloses are heteropolysaccharides commonly found in woody parts of plants, cereal straw with cellulose [32, 33]. Depending on the content of hemicelluloses of certain monosaccharides, they are called mannans, galactans or pentosans (xylans, arabans). The most important and common in nature are pentoses - arabinose and xylose, and especially hexoses - glucose, fructose, galactose, mannose. Hemicellulose can be split by xylase enzymes to form pentose sugars. Some Clostridia sp. synthesize xylanase and therefore are able to use xylan, a major component of hemicellulose, as an essential carbon source. Hemicelluloses are decomposed by three sets of enzymes - the first set includes xylanase, mannase, galactase, arabinase, the second and the third sets contain cellulase and β-glucanase, respectively [11, 14, 16, 17, 33].
Cellulose is a linear homopolysaccharide of β-D-glucopyranose in the form of long filaments joined by β-1,4-glycosidic bonds [33, 34]. Its structural unit is disaccharide cellobiose made up of β-glucose residues. The association of cellulose molecules in a stable crystalline structure led to the formation of microfibrils with 15 - 45 chains. At the microscopic level, there are microfibrils that form a complete fibril. Cellulose in the paracrystalline structure contains both amorphous and crystalline regions. The degree of polymerization is one of the main factors affecting cellulose bioconversion.
Cellulose is digested by cellulase, which acts to split molecules of cellulose into cellobiose and finally to glucose. Cellulose chains are very stable due to the interchain hydrogen and van der Waals interaction between pyranose rings that form crystalline regions [35]. The cellulose crystalline regions are interspersed with amorphous ones, which are degradable much easier. The cellulases responsible for the hydrolysis of cellulose are composed of a complex of proteins that have different specific activities for the hydrolysis of glycosidic bonds. Depending on their specific activities, cellulases are divided into three main classes: endoglucanases, cellobiohydrolases (exoglucanases) and β-glucosidases. Endoglucanases accidentally attack the cellulose chains in the unprotected positions of the amorphous region and create new ends, while the exoglucanases digest the polymer chain, both from the reducing and non-reducing ends, producing the main product - cellobiose. Endoglucanases exhibit high specific activity against soluble cellulose derivatives, such as carboxymethylcellulose, and low (or none at all) activity for microcrystalline cellulose as compared to exoglucanases. Synergistic action of both enzymes is required for the efficient cellulose degradation [35].
Lignin, an other component of lignocellulolytic biomass, has only recently attracted the interest of scientists. Lignin is a natural polymer that is located in cell walls and intercellular space and holds cellulose fibers [11, 14, 16, 17, 33, 36, 37]. Lignin can be isolated from plant tissues in various ways. It dissolves in both acids and alkalis. Protolignin is almost insoluble in organic solvents. Lignin, if isolated in different ways, differs in its composition and properties. In the chemical sense, lignin is a general and conditional concept - there can be no two fully identical lignins in nature. Numerous lignin formulas can be found in the literature [36, 37], but International Lignin Institute recommended to use the structural lignin formula [11]. As a rule, a lignin molecule is indefinitely large and contains multiple functional groups. A common structural constituent of all types of lignin is phenyl propane derivatives. According to the modern concepts, lignin is a complex three-dimensional polymer of aromatic nature, formed as a result of polycondensation of several monolignols - cinnamon alcohols (paracumarone, coniferyl and sinapic).
Modern methods of analysis allow to determine with sufficient accuracy the constituencies (individual macrocomponents) that are part of plant biomass [27]. In order to identify the carbon sources that are part of the biomass, the components of switchgrass (Panicum virgatum), rapeseed (Brassica napus), sweet sorghum (Sorghum saccharatum), wheat (Triticum aestivum), soybean (Glycine max) and paper mill sludge were determined Fig. (1) and published earlier [2, 27, 31, 38-40]. The green biomass of soybean, rape and wheat was provided by Institute for Agricultural Engineering and Electrification (National Academy of Agrarian Sciences of Ukraine), switchgrass and sweet sorghum - by M.M. Hryshko National Botanic Garden (National Academy of Sciences of Ukraine), components of switchgrass (cellulose, arabinogalactan and lignin) after thermobaric hydrolysis - by V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry (National Academy of Sciences of Ukraine) and paper mill sludge - by Kyiv Cardboard and Paper Mill.
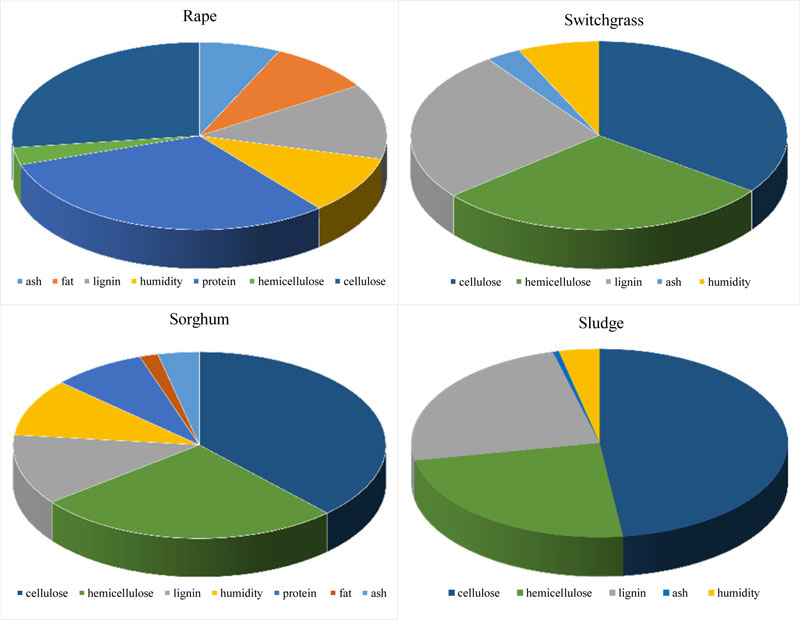
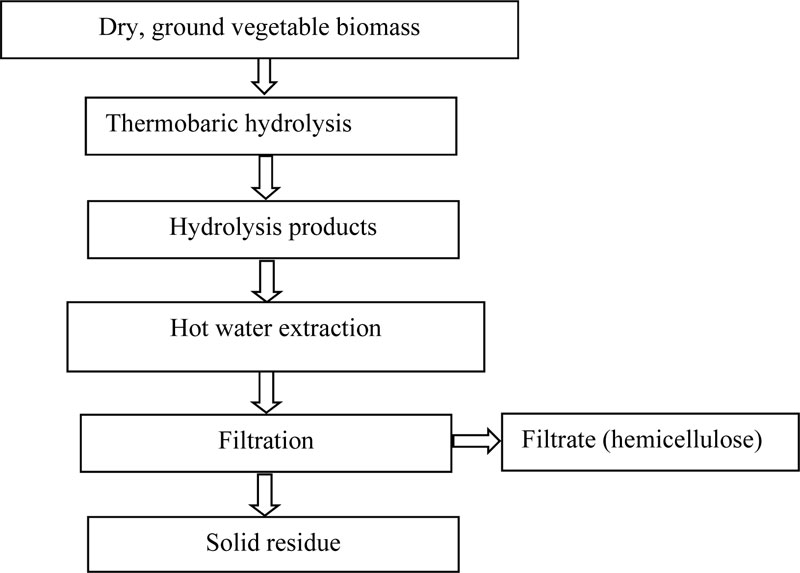
The thermobaric hydrolysis of lignocellulosic raw materials was performed with specially constructed equipment at a temperature of 200 0C for 10-15 min [31]. Dry ground switchgrass biomass (8% humidity) was placed in a reactor, some part of the water was added and hydrolysis was performed. The result was a mushy mixture, which was either immediately used as a substrate for the fermentation or fractionated according to the scheme shown in Fig. (2). Cellulose (35%), hemicellulose (29%) and lignin (26%) were found to be the main components of the biomass of switchgrass; for rape - protein (31%), cellulose (27%), hemicellulose (3%) and lignin (13%); for sludge - cellulose (48%), lignin (24%) and hemicellulose (24%) [31, 40]. The identified biomass composition allows to deduce that Clostridium bacteria are able to grow and convert the biomass of these species [2]. Therefore, in all these experiments, the butanol-producing strains C. acetobutylicum IMB B-7407 (IFBG C6H - parental strain) and Clostridium sp. IMB B-7570 (IFBG C6H 5M - mutant strain) from the collection of SI Institute of Food Biotechnology and Genomics (hereinafter referred to as IFBG Collection) were used.
3. PRODUCING STRAINS
Different strains of C. acetobutylicum absorb the vast majority of sugars present in the hemicelluloses of wood and its hydrolysates, in particular, xylose, which is the main component of hemicellulose. The bioconversion of xylose by C. acetobutylicum was investigated on dried grass [41-43]. The bioconversion of β-glucans and xylans was shown by C. acetobutylicum-6, C. acetobutylicum-7 and C. acetobutylicum VKPM B-4786 strains which had high hemicellulase activity. Such conversion increased with an increasing proportion of dry grass in the cultivation medium [44, 45].
Isolation of new strains of C. acetobutylicum is essential for efficient bioconversion of cellulosic waste. As reported previously [46-49], 20 strains of Clostridium spp. were screened for their cellulolytic activity and only two C. acetobutylicum strains, namely NRRL B527 and ATCC 824, demonstrated cellulolytic activity and the ability to produce extracellular enzymes. Previous studies [15] explored the possibility of producing solvents (butanol, methanol and ethanol) from cellulosic biomass using the C. acetobutylicum P 262 strain and suggested technology for butanol production from cellulose.
Some authors have also shown that the substrate composition influences the composition and enzymatic activity of C. cellulovorans cellulosome [50-52]. Bacterial genes encoding cellulase can be either randomly distributed, as in C. thermocellum, or clustered on the chromosome, as in C. cellulovorans, C. cellulolyticum and C. josui [53-57]. Other authors have identified the genes that encode putative cellulolytic components in a chromosome of C. acetobutylicum ATCC 824 strain [58].
To summarise our findings, it can be concluded that currently, the main obstacle to the commercialization of the cellulose enzymatic hydrolysis is the high cost of enzymes. This is further complicated by the fact that the low activity of cellulases requires a large number of lignocellulolytic enzymes [59]. In our view, the strategies to improve the economic feasibility of the hydrolysis of raw materials can be divided into four groups:
(1) Screening organisms with new enzymes [60-64];
(2) Improvement of existing industrial organisms and techniques for using their enzymes [65, 66];
(3) Improvement of operations related to substrate selection, cultivation conditions, enzyme processing and process reorganization [67-70] and
(4) Creation of genetically modified plant cultures [71].
Previous studies have shown that high hemicellulase activity observed in C. acetobutylicum-6, C. acetobutylicum-7 and C. acetobutylicum VKPM B-4786 strains made it possible to use plant biomass for the butanol production instead of corn or wheat flour as a traditional substrate [44]. Moreover, repeated “passages” of C. thermocellum 5 CT strain with high cellulolytic activity enabled the authors [60] to obtain thermostable enzymes.
Several studies were conducted earlier [72-80] to determine the ability of new strains to digest different components of lignocellulosic biomass. Clostridium cultures IFBG C6H and IFBG C7P were selected from the IFBG collection. They were introduced into the synthetic medium with sugars that are part of the hemicelluloses. It was shown that the selected strains can metabolise mannose, glucose, cellulose, arabinose and xylose. The maximum accumulation of butanol (1.9 g/l) was obtained by using xylose as a carbon source in the cultivation of IFBG C6H strain and cellulose with IFBG C7P strain (1.3 g/l). Similar results were obtained by the authors in the process of fermentation on model mixtures consisting of yeast extract and peptone with the sugars: cellulose, arabinose, glucose, xylose, cellobiose. Earlier research was conducted [76-83] to compare the butanol production efficiency of the above mentioned new strains with that of the old ones. The results demonstrated that the new strains could digest a wide range of raw materials. Due to the ability of these strains to dissimilate cellulose, their cultivation continued with cellulosic raw materials added to the medium.
To confirm the cellulose fermentation ability of IFBG C6H and IFBG C7P strains, the latter were plated on a filter paper. After 72 h of cultivation, transparent areas appeared on the filter paper, and after 120 h, the destruction of the paper around the colonies was observed. These tests demonstrated the bioconversion of non-food raw materials (filter paper) by 90% and 60% for IFBG C6H and IFBG C7P strains, respectively. On cellulose rich substrate, both strains produced acetic and butyric acids at a concentration of approximately 4 g/l and produced almost no alcohol. These results allowed to suppose the presence of cellulases in C. acetobutylicum IMB B-7407 (IFBG C6H - parent strain), Clostridium sp. IMB B-7570 (IFBG C6H 5M - mutant strain) and the possibility of using cellulose as a carbon source.
To study the ability of the strains of Clostridium sp. to assimilate lignocellulosic raw materials, a study was conducted by cultivating them on different lignocellulosic-rich biomasses/wastes (rape, soybean, wheat, switch grass, sawdust of various tree species, stock, fallen leaves). The dried green biomass was used without further pre-treatment. All strains assimilated the biomass and converted it to butanol Fig. (3). However, the accumulation of butanol was low (i.e., less than 1 g/l).
4. SUBSTRATE PREPARATION
Pre-treatment of lignocellulosic raw material is essential to facilitate its bioconversion. The pre-treatment is usually performed in several technological steps. After drying, the basic pre-treatment step for all types of lignocellulosic raw materials is grinding [84]. The procedures and extent of grinding determine the temperature regime of subsequent water-heat treatment and the extent of the fermented carbohydrates release. The higher the degree of dispersion of the raw materials, the lower the boiling point and sugar content, while the ABE solvents yield is increased. This is due to the partial cellulose degradation and the formation of additional fermentable sugars [2].
Effect of the variation of grinding degree of rape and switchgrass biomass on butanol accumulation by IMB B-7570 strain was investigated earlier [38-40]. Without plant biomass grinding, butanol accumulated at a concentration of up to 0.1 g/l. With prior grinding of rape biomass, butanol accumulation in the culture fluid increased, the highest level of butanol accumulation was observed at the grinding degree of 200 mesh (0.076 mm). We believe that the improvement in substrate bioavailability may be due to the fact that grinding decreases the number of crystalline cellulose zones and increases the number of amorphous zones, which are easily fermentable by the bacterial enzymes. In Clostridium bacteria, the enzymes that breakdown cellulose are part of the extracellular multiprotein complex - cellulosome.
A recent study was conducted to test the accumulation of butanol by ІМВ B-7570, IFBG C4B, and IFBG C6H strains using ground rape biomass as a substrate [27]. Three major products of the ABE process - acetone, butanol and ethanol - were identified in the fluid of these cultures. The highest accumulation of butanol (2.3 g/l) was shown for Clostridium sp. ІМВ B-7570 strain; acetone was present in a small amount (0.5 g/l), and ethanol in the amount of 0.1 g/l. The cultivation of Clostridium sp. ІМВ B-7570 (IFBG C6H 5M), C. acetobutylicum IIB B-7407 (IFBG C6H) and C. tyrobutylicum IFBG C4B strains on sorghum biomass, juice and bagasse was also performed. It was shown that the least accumulation of butanol (0.5 g/l) was achieved by C. tyrobutylicum IFBG C4B on ground sorghum biomass, whereas the highest accumulation (8.2 g/l) by C. acetobutylicum ІМВ B-7407 (IFBG C6H) on sorghum juice.
One of the possible subsequent lignocellulosic biomass pre-treatment steps is the temperature treatment to release sugars. This treatment can be carried out either at a relatively low temperature (135-145°C) for one or more hours or at a higher temperature (165-175°C) for 2 to 5 min, or in a stage wise manner - from higher temperature to lower [84]. The raw material preparatory treatments can be conducted in a variety of ways, for example with organosol [85, 86], alkali [87], sodium hydroxide, acid [88, 89], ionic liquid [90-92], high-frequency heating [93], sharp steam [94, 95], lime [96] or ammonia [97]. The most promising of them is Organosol preparation and treatment with the help of acute steam. Such processing increases the sugar yield, reduces the amount of insoluble lignin and increases the content of easily fermentable cellulose [85, 86].
Another possible step of raw material preparation is through the hydrolysis of sugars to pentoses or hexoses. Such hydrolysis can be performed with the use of alkalis, acids or enzymes. Enzymatic hydrolysis involves the use of both enzymes and enzyme-producing organisms. The catalytic activity of enzymes is characterized by “turnover number”, that is, the number of moles of substance converted per time unit per enzyme mole. The activity of enzymes depends on temperature, concentration of water ions, presence of activators and inhibitors. The detailed mechanism of lignocellulose enzymatic hydrolysis has not yet been elucidated.
Significant progress has been made in the study of lignocellulolytic genes of organisms known to show hydrolytic properties (including bacteria and fungi) [98, 99]. Currently, more than 14,000 mushroom species are known to exhibit cellulolytic activity, and only some of them are used in industrial technologies [100]. Trichoderma reesei is one of the most common organisms for cellulose and hemicellulose degradation, although it does not have enzymes for lignin degradation [98-100]. The major role in lignin processing is played by basidiomycetes, living on both live and deadwood. Much of protolignin is degraded by brown rot fungi, which cause the hydrolysis of polysaccharides [11]. The effective destructors of lignin are white rot fungi, among which Phanerochaete chrysosporium is studied the best [101, 102]. These are species used in industrial technologies for the production of lignin degrading enzymes, as well as for direct use during the lignocellulose bioconversion [103, 104].
Polymeric lignin degradation occurs under the action of extracellular enzymes - oxidoreductases, in particular ligninperoxidase, Mn-peroxidase and laccase. In addition, the ligninolytic fungi complex includes pyranosoxidases, glucose oxidases, glyoxal oxidase and cellobiodehydrogenase. Daedalea flavida, Phlebia fascicularia, P. floridensis and P. radiate fungi have not been studied deeply enough and they are used only for the lignin separation from raw materials and for the selective lignin degradation [105]. Cellulomonas, Pseudomonas, Thermomonospora and Microbispora actinomycetes, as well as bacteria with a surface-bound cellulose complex, such as Clostridium thermocellum and Ruminococcus, have a slight lignin digestion potential [106]. Organisms with high lignin degradation activity could be used as a source of the gene pool for engineering those organisms with slight lignin digestion potential. Currently, the cost of enzymes is one of the major obstacles to exploit the hydrolysis of cellulose by an enzymatic approach. Due to the low cellulase activity of enzymes, it is necessary to increase the bioavailability of cellulose (destroy its crystalline regions) by mechanical treatment [107] or hydrolysis.
Fractionation of the lignocellulosic switchgrass biomass onto its components following the thermobaric treatment produces water-soluble substances (Table 1) and stock (Table 2). The feasibility of using switchgrass lignocellulose components as substrates for butanol synthesis was tested by cultivation of the IFBG C6H and IFBG C6H 5M strains on various switchgrass components and sludge medium [31].
It was found that the products initially obtained with the thermobaric hydrolysis of switchgrass need to be purified from furfural, formed during the thermobaric treatment and that acts as an inhibitor of the microorganisms. It was also shown that switchgrass sludge contains a higher proportion of cellulose in comparison to the switchgrass biomass [31]. From the results, it is clear that the obtained components (cellulose, arabinogalactan, lignin) can be used to produce butanol. It should be noted that, due to the sludge thermobaric hydrolysis, furfural was formed in a small amount, which did not affect the viability of the microorganisms and did not require additional separation. In this case, the use of thermobaric hydrolysis increased the accumulation of target product twofold [31].
| Components | original Biomass, % | Biomass after autohyrolysis, % |
|---|---|---|
| Cellulose | 46,7±0,5 | 53,9±0,5 |
| Hemicellulose | 23,0±1,0 | 10,6±1,0 |
| Soluble substances | 7,7±0,5 | 11,5±0,5 |
| Lignin | 13,8±0,2 | 14,7±0,2 |
| Resins and fats | 2,0±10 | 2,0±10 |
| Ash | 5,4±0,5 | 5,4±0,5 |
| Others | 1,4±0,2 | 1,9±0,2 |
| Total | 100,0 | 100,0 |
| Components | Original composition, % | Composition after autohyrolysis, % |
|---|---|---|
| Cellulose | 34,0±0,5 | 37,0±0,5 |
| Hemicellulose | 3,7±1,0 | 1,5±1,0 |
| Soluble substances | 3,2±0,5 | 6,9±0,5 |
| Lignin | 8,5±0,2 | 3,5±0,2 |
| Resins and fats | 2,3±10 | 2,3±10 |
| Ash | 47,8±0,5 | 48,0±0,5 |
| Others | 0,5±0,2 | 0,8±0,2 |
| Total | 100,0 | 100,0 |
5. OPTIMIZATION OF CULTIVATION PARAMETERS
An important aspect of the ultimate cost of butanol production is also the cost of the substrate and its concentration in the fermentation medium. The efficiency of butanol accumulation using different concentrations of switchgrass and rape biomasses in the fermentation medium was investigated earlier [27, 38]. The data showed that the accumulation of butanol in the culture fluid increased with the increase of the rape biomass concentration from 5 to 10 g/l. Further increase in the amount of dry ground rape biomass from 15.0 to 30.0 g/l decreased the accumulation of butanol. The results of study showed that the bioavailability of the substrate decreases with the increase in carbon concentration. The highest accumulation of butanol (2.9 g/l) in the culture fluid was obtained with a dry ground rape biomass concentration of 10.0 g/l. and the raw materials pre-treated by autoclaving for 2 hours at a pressure of 2 atm. Further increase in the processing time or pressure did not significantly affect the production of butanol.
However, carbon is not the only essential parameter affecting the growth of microorganisms. It is known that optimization of cultivation parameters such as temperature, pH and incubation time can increase the accumulation of butanol in cultural fluid several times [108, 109]. The influence of cultivation parameters on butanol accumulation was investigated, using switchgrass as a substrate. The effect of temperature of cultivation on butanol accumulation was investigated on IFBG C6H and IFBG C7P strains. The basic cultivation parameters of these strains were optimized, as described earlier [38]. It was shown that the temperature increase from 28±1oC to 36±1oC led to an increase in the butanol accumulation for both strains, temperature increase to 38±1oC reduced the butanol yield in the culture fluid. All further studies were performed at 35±1oC.
It is well known that the pH of the medium significantly affects butanol accumulation. In our experiments, the effect of pH on butanol production was investigated using the IFBG C6H and IFBG C7P strains. It was found that the maximum butanol accumulation (3.5 g/l) occurred at pH 7.0 with IFBG C6H strain and 2.5 g/l at pH 6.0 with IFBG C7P strain. Moreover, pH adjustment from 5 to 7 did not affect butanol accumulation by the IFBG C7P strain. At the beginning of the fermentation, the bacterial count was determined by the quality and quantity of the bacterial inoculum. The number of cellulosomes increased following the increase of bacterial count in the fermentation medium. For this reason, to standardize the amount of bacteria introduced into the inoculum, the liquid nutrient media with water-soluble carbon sources were used.
The effect of the seed material concentration on butanol accumulation was investigated using lignocellulosic biomass as a substrate and the IMB B-7570 strain. It was established that with the increase of the seed material concentration from 5 to 10% of the fermentation mixture volume, the butanol synthesis increased [2]. The increase of inoculum concentration up to 15-20% caused a negative effect on butanol accumulation. Further increase in seed material concentration added to the fermentation mixture generally suppressed the butanol synthesis in the medium. The inhibition of butanol synthesis by the high inoculum concentration may be due to an increase of the primary metabolites of the ABE fermentation (butyric, lactic and acetic acids) in the accumulation medium. The optimum concentration of the inoculum introduced into the fermentation medium was 10%. Under such conditions, the largest amount of butanol accumulated was 2.5 g/l.
Since ABE is a biphasic process, the time of cultivation is very important for optimisation of butanol accumulation conditions. The impact of changing cultivation times on butanol accumulation in the fluid was investigated using IFBG C6H and IFBG C7P strains. The maximum butanol accumulation was obtained after 72 h of cultivation for both IFBG C6H and IFBG C7P strains on a synthetic medium. Further elongation of the cultivation time did not produce positive effects on butanol synthesis. Optimization of the technological parameters by testing different types of plant material allowed to increase the butanol accumulation by 20% (2 g/l) with strain IFBG C6H and by 50% (4.3 g/l) with the mutant strain IFBG C6H 5M [40].
6. USE OF SYNTHESIS PRECURSORS
ABE fermentation is known to be a type of butyric acid fermentation; therefore, the addition of precursors of this process, such as lactic, acetic and butyric acids, to increase the accumulation of butanol, has been investigated.
The corresponding acids were introduced into the enzymatic medium, first individually, and then in the mixture, and for each case, the cultivation was performed [40]. The obtained data indicate that the use of synthesis precursors increased butanol accumulation. Slight stimulation of butanol accumulation, as compared to control (3.5 g/l), was observed in the case of acetic acid (3.7 g/l) and serum (3.9 g/l). A significant 1.7-fold increase in butanol concentration was due to the use of a mixture of all three precursors, with the conversion of sugars almost doubling from 32% to 58%. The addition of precursors to the fermentation medium increased the economic coefficients of fermentation, but did not affect biomass accumulation and specific growth rate of culture. It should be noted that the ratio of butanol concentration to biomass increased from 1.2 to 2 with the use of precursors. At the same time, the biomass to consumed substrate ratio decreased from 0.38 to 0.29, respectively. Such a change in kinetic parameters as a result of precursors addition indicates an increase in butanol accumulation due to substrate conversion. To determine the optimal concentration of precursors, the cultivation with different combinations of concentrations of precursors was performed. The butanol synthesis precursors introduced into the fermentation medium increased the final concentration of end-product differently. The optimal option was a mixture of lactic, acetic and butyric acids in a ratio of 1: 1: 3, respectively. The addition of a mixture of these acids increased the concentration of the target product by 17% [40].
7. WASTE DISPOSAL
An important issue related to butanol production technology is the production of waste disposal. The utilization (evaporation) of the acetone-butyl bard is quite costly. To overcome this challenge, we considered an option to return the acetone-butyl distillery waste to the cultivation process [40]. The use of “reverse” distillery waste to reduce production waste was successful. The concentration of the reverse distillery wastes up to 60% of the volume of the fermentation mixture did not significantly affect the synthesis of butanol by C. acetobutylicum IFBG C6H 5M strain. Further increase in the concentration of distillery waste in medium decreased accumulation of butanol. This finding makes it possible to return up to 60% of the distillery waste to the enzymatic medium. The remaining part of the distillery waste was separated into decantate and sludge. The decantate contained 80-85% of the distillery waste, 2% of solids, 0.7 g/l of reducing substances and had a pH 4. The decantate was used instead of water in further fermentation (“reverse” water), and the sludge can be used as animal feed or as a fertilizer [40].
CONCLUSION
Overall, our studies demonstrate the possibility to produce butanol efficiently from plant biomass. The accumulation of solvents (alcohols) directly depends on the characteristics of the butanol-producing bacterial strains and the stages of biomass pre-treatment (preparation) - drying, grinding, sterilization, type of hydrolysis. In order to develop commercial biobutanol technology using lignocellulosic raw materials, it is, however, necessary to improve significantly the efficiency of the bioconversion of lignocellulosic raw materials by selecting high yield bacterial strains and optimizing the efficiency of pre-treatment methods of the biomass.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors acknowledge hereby the support provided in part of project “Creating effective butanol producing strains capable of fermenting bio-raw materials with an increased yield of target product” (no. 0111U001489), “Improvement of biobutanol technology using alternative substrates and domestic producing strains” (no. 0113U005527), ”Improvement of butanol production technology based on rapeseed biomass using domestic bacteria producing strains” (no. 0118U005353).


