All published articles of this journal are available on ScienceDirect.
A Rapid Bioassay to Evaluate Efficacy of Hypovirulent Binucleate Rhizoctonia in Reducing Fusarium Crown and Root Rot of Tomato
Abstract
Background:
Fusarium Oxysporum f.sp. Radicis-Lycopersici (FORL) caused Fusarium Crown and Root Rot of tomato (FCRR), it’s a serious constraint on tomato production and contributing to yield losses.
Aims/Method:
Using a rapid bioassay, Hypovirulent Binucleate Rhizoctonia (HBNR) was tested for their ability to reduce Fusarium Crown and Root Rot (FCRR) of tomato, caused by Fusarium oxysporum f.sp. radicis lycopersici (FORL). Roots of tomato seedlings growing on 2% water agar in plastic boxes were inoculated with living or dead mycelial disks of HBNR. After 24 h, the pathogen was applied at 0, 3, 6, and 9 cm away from the position of the HBNR.
Results:
When living HBNR was used, the treatments provided significant protection to tomato seedlings from FCRR infection at all distances tested. Tomato plants pre-inoculated with living HBNR at different times (12 h and 24 h before inoculation with the pathogen) and challenged with FORL showed significant reduction of FCRR lesion development. A significant reduction was still observed even when HBNR was inoculated simultaneously with or 12 h after inoculation of a pathogen. Seedlings treated with dead HBNR and culture filtrates also showed significantly reduced FCRR lesion development. When living HBNR were enveloped by a polycarbonate membrane filter, a significant reduction of FCRR lesion development was still observed.
Conclusion:
In all experiments, reduction of FCRR lesion development in seedlings treated with HBNR tended to decrease with longer distance from the inoculation point of FORL and HBNR. We developed a simple, rapid, and miniaturized bioassay for evaluating the efficacy of HBNR against FORL. The bioassays require only 12 - 18 days, which is at least 12 days less than the soil system employed by previous researchers.
1. INTRODUCTION
Fusarium Crown and Root Rot of tomato (FCRR), caused by Fusarium Oxysporum f.sp. Radicis-Lycopersici (FORL), is a serious constraint on tomato production that limits the yield of greenhouse- and field-grown tomato crops [1]. The disease was first detected in Japan in 1974 [2]. Yield losses caused by FCRR in greenhouse and field tomato production range from 15 to 65% [3].
Recent research on the management of Fusarium wilt and FCRR has focused on diverse strategies, either individually or in combination. These strategies include host resistance and chemical, biological, and physical control [4]. Vitale et al. [5] demonstrated that grafting tomato hybrid plants onto “Natalia” rootstock significantly enhanced the tolerance of plants to FORL, even though proteomic analysis showed a higher representation of proteins associated with pathogen infection. A combination of a plant-growth-promoting strain of Fusarium equiseti with biodegradable pots was also an effective control of FCRR [6].
Hypovirulent Binucleate Rhizoctonia (HBNR) were investigated as effective biocontrol agents for a number of important diseases caused by Rhizoctonia solani [7] and Phytium [8]. Our previous research showed that HBNR effectively controls Fusarium wilt of tomato [9], Fusarium wilt of spinach [10], and Fusarium crown and root rot of tomato [11]. These studies indicated that one of the mechanisms of biocontrol of fusarium diseases with HBNR isolates might be induced resistance. Investigations of HBNR as an agent of Induced Systemic Resistance (ISR) in beans, against the root rot pathogen Rhizoctonia solani or the anthracnose pathogen C. lindermuthianum, have also been reported [12]. HBNR also effectively protected cotton seedlings against rhizoctonia damping-off and Alternaria leaf spot with a mechanism of Induced Systemic Resistance (ISR) [13].
A major limiting factor in the development of biological control strategies for different plant diseases is the formulation of efficient procedures for rapidly screening large numbers of organisms for biological control activity. While field screening should theoretically provide the best detection of efficient biocontrol strains, limitations of space, labor, cost, and optimal environmental conditions preclude the use of this type of screening strategy. Laboratory assays based on the in vitro inhibition of pathogens or production of particular metabolites by biological control agents offer a rapid and relatively inexpensive means of screening organisms but may not be good indicators of biocontrol potential. Unsurprisingly, biocontrol strains selected in vitro on the basis of phenotypes with unknown links to biological control activity in plant systems do not always perform as expected under greenhouse or field conditions [14, 15]. The present study was undertaken to: (1) develop a rapid and miniaturized laboratory bioassay for screening the efficacy of HBNR in reducing FCRR in the tomato; (2) investigate the efficacy of various inoculum forms (living and dead mycelial disks) of HBNR in controlling FCRR using a water agar system.
2. MATERIALS AND METHODS
Organisms: Four isolates of HBNR were used as biocontrol agents: L2 (AG-Ba), W1, W7 (AG-A), and HBNR Rhv7 (unknown anastomosis group). Fusarium Oxysporum f.sp. Radicis-Lycopersici (FORL) isolate RJNI, obtained from a tomato infested with Fusarium Crown and Root Rot (FCRR), was used as the inoculum of the pathogen.
Plant: Tomato cv. “House Momotaro”, a popular cultivar that is susceptible to FCRR, was used throughout the experiments.
Inoculum preparation: (1) The pathogen, FORL, was grown on Potato Dextrose Agar (PDA) for 7 days in the dark at 25oC. Spores were scraped from the cultures with a sterile glass bar, and a spore suspension was prepared in sterile water and filtered through eight layers of sterile gauze. (2) HBNR isolates were prepared as inoculum forms in Potato Dextrose Agar (PDA) plugs (living and dead mycelial disks). The isolates were grown on PDA for 3-7 days in the dark at 25 oC. The dead mycelial disk was prepared by killing the 7-day-old culture with chloroform and then drying it for 60 min on a clean bench. To make Culture Filtrate (CF), two mycelial disks of each HBNR isolate, obtained from the growing margin of a colony on PDA, were transferred to a 200-ml flask containing 50 ml of potato dextrose broth (pH 6.5). The isolates were cultured without shaking for 10 days in dark. The crude culture filtrate was separated from mycelia and filtered three times through three layers (each time) of Whatman no. 2 filter paper. The CF was then filter sterilized (0.45-μm Millipore filters, Millipore Products Division, Bedford, USA).
2.1. Laboratory Assay of Biological Control of Fusarium Crown and Root Rot of Tomato
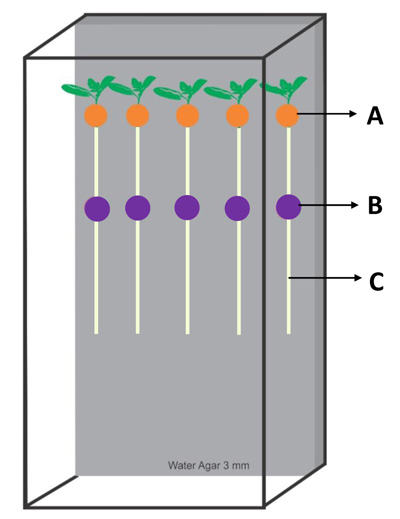
The efficacy of HBNR in suppressing the development of FCRR in the tomato was tested in laboratory experiments using a Water Agar (WA) system method (Fig. 1). Tomato seeds were surface-sterilized in 70% ethyl alcohol for 1 min followed by soaking in 1% sodium hypochlorite with 3 drops of Tween 20 (polyoxyethylene sorbitan monolaureate; Nacalai Tesque, Inc., Kyoto, Japan) for 20 min. The seeds were then rinsed three times with Sterilized Distilled Water (SDW). The seeds were pre-germinated on 2 layers of Whatman No. 1 filter paper for 3 days in the dark at 25oC. Five seedlings were transferred to a sterilized plastic box (196 × 104.5 × 28 mm) containing Water Agar (WA) and allowed to grow for 6 days at about 20 in a cleanroom. A living HBNR mycelial disk (3-mm diameter, taken from the advancing margin of a three-day-old culture), a dead mycelial disk (7-mm diameter), and CF (70 µl) were used to inoculate the basal hypocotyls of the seedlings, which were again incubated for 24h. To prevent, spread and maintain a uniform distribution of CF on basal hypocotyls or roots, drops of CF were placed on an 8-mm diameter paper disc with 1.5-mm thickness (Advantec, Toyo Roshi Kaisha, Ltd. Japan). To avoid direct contact between HBNR and FORL, the mycelial disk of HBNR was enveloped by a polycarbonate membrane filter (0.2-µm mesh). An additional experiment using a living mycelial disk (3-mm diameter) without an enveloping membrane was also done. In this experiment, the inoculation period of the HBNR varied from 0 h to 12 h after inoculation with the pathogen and 0 h to 24 h prior to inoculation with the pathogen. As a control, seedlings were inoculated with HBNR-free PDA or SDW. Then, 5 µl of pathogen suspension (5 × 105 spores/ml) were inoculated at positions 0, 3, 6, and 9 cm away from the position of the HBNR inoculum. A 5-mm diameter disk of lens paper was placed on each drop to prevent runoff and to maintain a uniform distribution of spores on the root surface. The treatments were prepared in four replicates. Treated and control seedlings were maintained at about 20oC for another 2-8 days. Disease severity was determined by measuring lesion development at the pathogen inoculation point. Percent reduction of lesion development was used to measure the efficacy of HBNR against the pathogen, by employing the formula [(A-B)/A] × 100, in which A represents the lesion length observed on the root due to inoculation of pathogen alone and B is the lesion length observed on the root due to co-inoculation of HBNR and the pathogen.
3. RESULTS
3.1. Biological Control of FCRR of Tomato with HBNR
In a WA system, tomato seedlings treated with living mycelia, dead mycelia, and CF of HBNR isolates significantly reduced lesion development of FCRR (P = 0.05).
When living mycelia were used as treatment, seedlings treated with HBNR isolates had significantly less FCRR lesion development after 4-8 days of pathogen inoculation (Fig. 2). The percentage of reduction tends to decrease with the longer distance between HBNR and FORL. At a distance of 0 cm between HBNR and FORL, the reduction of lesion development by HBNR L2, W1, W7, and Rhv7 was almost completely ranged from 88-98%. At a distance of 3 cm, application of all HBNR still highly reduced lesion development by 88-96%. At a distance of 6 cm and 9 cm, the reduction of lesion development by all HBNR isolates slightly decreased by 55-94% and 11-66%, respectively (Fig. 2).
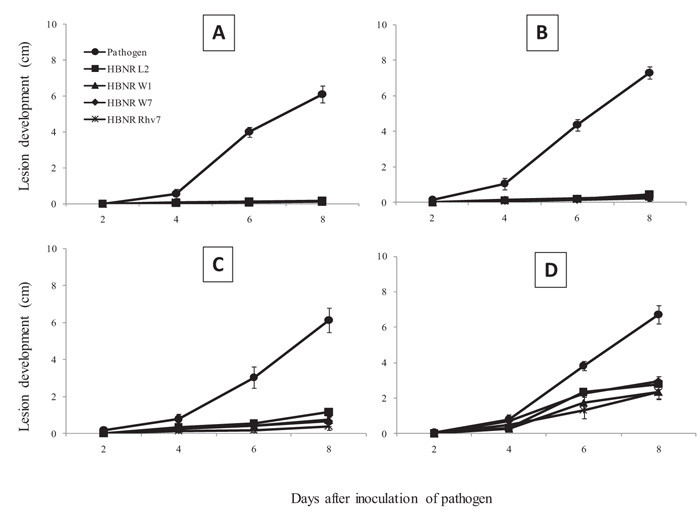
Tomato seedlings treated with dead mycelia of all HBNR isolates except L2 also showed significant reduction of FCRR lesion development 2-8 days after inoculation with the pathogen (Fig. 3). At a distance of 0 cm, lesion development reduction was 6-21%, 22-79%, 9-49%, and 4-52%, for HBNR L2, W1, W7, and Rhv7, respectively (Fig. 3A). At a distance of 3 cm, the reduction of lesion development by HBNR L2, W1, W7, and Rhv7 was 5-37%, 16-52%, 10-41%, and 9-59%, respectively (Fig. 3B). At a distance of 6 cm, lesion development reduction was 2-34%, 15-45%, 10-49%, and 4-48%, respectively (Fig. 3C).
The application of CF of HBNR isolates also resulted in significant reduction in FCRR lesion development 2-8 days after pathogen inoculation (P = 0.05; Fig. 4). At a distance of 0 cm, the reduction of lesion development by HBNR L2, W1, W7, and Rhv7 was 35-85%, 36-73%, 37-64%, and 36-78%, respectively (Fig. 4A). At a distance of 3 cm, treatment with HBNR L2, W1, W7, and Rhv7 reduced lesion development by 30-79%, 31-83%, 23-74%, and 27-88%, respectively (Fig. 4B). At a distance of 6 cm, the reduction of lesion development by HBNR L2, W1, W7, and Rhv7 was 30-70%, 33-72%, 26-84%, and 27-86%, respectively (Fig. 4C).
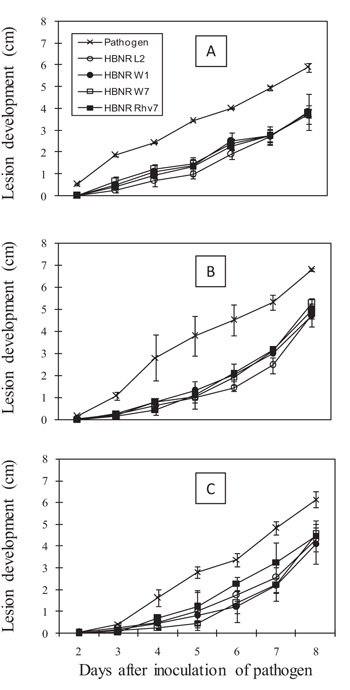
We attempted to prevent direct contact between HBNR and FORL by enveloping the living mycelia in a polycarbonate membrane filter (0.2-μm mesh), but the mycelia still penetrated the membrane, so that direct contact between HBNR and FORL was observed. In this experiment, a significant reduction in FCRR lesion development was still observed up to 8 days after pathogen inoculation at a distance of 0 cm (P = 0.05; Fig. 5A). At a distance of 3 cm, a significant reduction in FCRR lesion development was observed until 6 days after pathogen inoculation (P = 0.05; Fig. 5B). However, at a distance of 6 cm, a significant reduction was only observed at 3-4 days after pathogen inoculation (Fig. 5C). The reduction of lesion development by HBNR W1 was 25-78%, 13-67%, and 10-52% at distances of 0, 3, and 6 cm, respectively.
In another experiment, pre-inoculation at 12 h and 24 h with living mycelia of HBNR W1 or Rhv7 on the seedlings, and challenge-inoculation with FORL at 3 cm and 6 cm away from HBNR, also resulted in significant reduction in lesion development compared to the control, after 8 days of pathogen inoculation (Table 1). At 12 h pre-inoculation of HBNR, at a distance of 3 cm, treatment with HBNR W1 and Rhv7 reduced FCRR lesion development by 90% and 91%, respectively. At a distance of 6 cm, the reduction by HBNR W1 and Rhv7 was 71% for both. The reduction slightly increased with the longer pre-inoculation period of 24 h. At a distance of 3 cm, the reduction by HBNR W1 and Rhv7 was 93% and 90%, respectively. At a distance of 6 cm, the reduction by HBNR W1 and Rhv7 was 82% and 74%, respectively. HBNR isolates also significantly reduced lesion development of FCRR (P = 0.01) when both isolates were applied simultaneously (0 h) and even when HBNR was applied 12 h after pathogen inoculation. At 0 h or simultaneous inoculation, at a distance of 3 cm, the reduction of lesion development by HBNR W1 and Rhv7 was 89% and 90%, respectively. At a distance of 6 cm, the reduction was 71% and 64% for HBNR W1 and Rhv7, respectively. At 12 h after pathogen inoculation, at a distance of 3 cm, the reduction was 89% and 81% for HBNR W1 and Rhv7, respectively. At a distance of 6 cm, the reduction by HBNR W1 and Rhv7 was 66% and 59%, respectively.
| Treatments | Lesion Development (cm)b | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 cmc | 6 cm | |||||||
| -12d | 0 | 12 | 24 | -12 | 0 | 12 | 24 | |
| Pathogen | 7.2 be | 7.0 b | 6.7 b | 7.0 b | 6.4 b | 6.1 b | 5.6 b | 6.1 b |
| HBNR W1 | 0.8 a | 0.8 a | 0.7 a | 0.5 a | 2.2 a | 1.8 a | 1.6 a | 1.1 a |
| HBNR Rhv7 | 1.4 a | 0.7 a | 0.6 a | 0.7 a | 2.6 a | 2.2 a | 1.6 a | 1.6 a |

4. DISCUSSION
In this study, all HBNR isolates tested using various inoculum forms, i.e. living mycelia, CF, and dead mycelia significantly reduced lesion development of FCRR. Maximum protection occurred when the pathogen was inoculated at the position of 0 and 3 cm away. However, protection decreased at a distance of 6 and 9 cm. In our study using the WA system method, the phenomena lesion development affected by biological control agents could be rapidly recorded without destructive to the root system. Living mycelia showed a stronger inhibition of lesion development throughout the experiment, while dead mycelium inhibited effectively lesion development up to 5 days then decrease at a longer time of incubation. It might be that on living mycelia, three were a competition in infection site between HBNR and FORL. HBNR has been reported to be an effective colonization of plant root [11, 16] and it was likely that inoculated living HBNR mycelia had been already colonizing the infection site that allows competition between HBNR and FORL. Pre-inoculation of living mycelia of HBNR at a different time, and challenged with FORL, resulted in significant reduction in FCRR lesion development, even when HBNR was inoculated simultaneously (0 h) or 12 h after inoculation of the pathogen.
Tomato seedlings treated with CF and dead mycelia of HBNR effectively reduced FCRR lesion development. The in vitro interaction experiments using living or dead mycelia and CF reveal that they did not produce any zone of inhibition (data not shown), suggesting that they were not antagonistic and ruling out the possible involvement of toxins or antifungal compounds in disease suppression. Since CF and dead mycelia of HBNR application sites and pathogen application sites were spatially separated by a distance of 3-6 cm, and there was no contact between HBNR isolates and the pathogen until day 5 at 3 cm and day 8 at 6 cm, we observed that average mycelial growth of the pathogen was 0.54 cm/day. Induced resistance in tomato plants by HBNR may be one of the mechanisms of biological control against FCRR in this study. These results confirm those of [17] and [18], who reported that HBNR did not inhibit or parasitize R. solani. Plant protection by hypovirulent binucleate Rhizoctonia involves resistance pathways such as Systemic Acquired Resistance (SAR), Induced Systemic Resistance (ISR), and phytoalexins [16].

Many reports demonstrated that mycelia or CF of fungi were effective in inducing resistance against various diseases [19-22] which further demonstrated that tomato plants treated with oligandrin, the elicitin-like protein produced by the mycoparasite Pythium oligandrum, showed significant induction of systemic resistance against FORL. The most striking features of the resistance mechanism involved restriction of fungal growth to the outer root tissues, a decrease in pathogen viability, and formation of aggregated deposits, which often accumulated at the surface of invading hyphae. In addition [23], reported that cucumber seedlings treated with pectinases extracted from fermentation products of Penicillium oxalicum BZH-2002 induced resistance against scab caused by Cladosporium cucumerinum.
Various bioassays for screening biocontrol agents use soil systems [9, 11, 24], and other bioassays for induced resistance in tomato plants have been reported, such as split root, benomyl, cutting, and layering [25]. However, these systems, like most other biocontrol assay, often require more than one month to complete. Such long-term bioassays are difficult to use in large screening trials. In contrast, the bioassay used in this study offers the advantage of a short assay period (12-18 days) and requires only a small amount of space in a clean room to test many different strains or isolates. Another advantage of this assay was its simplicity and the need for only small amounts of biocontrol agent and pathogen inoculum. By screening strains initially on plants, as opposed to pathogen-inhibition assays in Petri dishes, we hope to minimize the erroneous selection of strains on the basis of biological control traits that would not be expressed in more complex ecosystems.
The results presented in this study establish that this rapid bioassay can also be effective to screen large numbers of microorganisms as biocontrol agents and plant resistance inducers. We expect that the bioassay used in this study could also be used as a rapid assay in pathogenicity testing of FCRR.
CONCLUSION
The laboratory assay developed in this study could rapidly be determined as biocontrol efficacy of HBNR against FCRR within 12-18 days from seedling emergence. Except for isolate L2, all isolates exhibited a strong and consistent biocontrol efficacy. Living mycelia were the most effectively used as a biocontrol inoculum, followed by CF, and dead mycelia.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable
FUNDING
We thank the Ministry of Education, Science, Sports, and Culture (Monbukagakusho) Japan, for financial assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


