All published articles of this journal are available on ScienceDirect.
Inhibition of Mycelial Growth of Rhizoctonia Solani by Chitosan in vitro and in vivo
Abstract
Objective:
Evaluate the antifungal effect of chitosan against Rhizoctonia solani in vitro and the possible mechanisms of its induced activity in potato tubers to control black scurf disease.
Methods:
The in vitro influence of chitosan at different concentrations on mycelial growth of R. solani was tested by using the poisoned food technique in PDA medium. The effect of these concentrations on the development of lesion diameters in tubers inoculated with R. solani mycelium was assayed for 30 days. The concentration that showed the greatest inhibitory effect on lesion diameters was tested to assess the induced activity of defense-related enzymes in the infected tubers.
Results:
In the poisoned food technique, chitosan at 1% completely inhibited the growth of R. solani mycelium. In vivo tests showed that chitosan treatment at 0.5% effectively controlled the black scurf in tubers inoculated with R. solani mycelium. Chitosan increased the activities of defense-related enzymes such as Peroxidase (POD), Polyphenol Oxidase (PPO) and Phenylalanine Ammonia-lyase (PAL) in treated tubers of tested cultivars.
Conclusion:
This work demonstrated that chitosan directly inhibited the growth of R. solani, and potentially elicited defense reaction in potato tubers.
1. INTRODUCTION
Rhizoctonia solani Kühn [teleomorph: Thanatephorus cucumeris (Frank) Donk] AG-3 PT, the pathogen of stem canker and black scurf in potato (Solanum tuberosum L.), is a worldwide plant disease [1]. Yield losses from R. solani can reach 50%, resulting in marked economic losses for farmers [2]. Control of R. solani relies mainly on fungicides application [3] such as azoxystrobin, flutolanil, and pencycuron [4, 5] which are not always efficient due to the development of fungicide resistant communities. Furthermore, pesticides are environmental and human health concern. Consequently, more momentum has gained in search of sustainable solutions to chemical control. Chitosan (CH) (poly-β-(1 4) N-acetyl-d-glucosamine) is a modified, natural safe biopolymer derived by deacetylation of chitin, the second most abundant natural polymer in the world [6, 7]. Chitosan is very useful for several industries, such as cosmetology, biotechnology, food, pharma- cology and medicine [8, 9]. Since the 1980s, farmers have used chitosan as biopesticide, biofertilizer and agricultural film in seed and fruit coating [10]. Chitosan has been demonstrated to control postharvest diseases on several horticultural commo- dities such as apples, pears, kiwifruit, strawberries, tomato, raspberries, table grape and others [11-15]. Chitosan protects rice, tomato, tobacco, and lettuce plants against R. solani infection [16-19]. However, to our recent knowledge, there is no available information about the antifungal activity of chitosan against potato black scurf pathogen R. solani. This study aims to evaluate the antifungal activity and the induced effect of chitosan treatment on potato tuber resistance to R. solani under in vitro and in vivo conditions.
2. MATERIALS AND METHODS
2.1. Potato Tubers
Tubers of cultivars Sante and Kolobok were harvested from Moiseev farm, Bazarno-Karabulaksky District, Saratov region, Russia. The tubers based on size and showing no visible signs of disease infection or physical injuries were packed in net bags, transported to the laboratory, and stored at (16 ± 2) °C. Before treatment, the tubers were superficially disinfected with 2% sodium hypochlorite for 3 min, washed several times with sterilized water to wash out the remaining disinfectant solution and then air-dried.
2.2. Pathogen
Tubers with typical symptoms of black scurf were used to isolate the pathogen. Diseased tissue was cut into small pieces (1 cm2) and surface-disinfected in 70% ethanol for 30 s, followed by treatment with 2% (vol/vol) sodium hypochlorite for 3 min. Superficially sterilized pieces were rinsed several times in sterilized water to wash out the remaining disinfectant solution and cut aseptically into two pieces. These pieces were then dried between sterilized filter papers and then were placed on Petri dishes containing fresh Potato Dextrose Agar medium (PDA) (Difco Laboratories, St. Louis) supplemented with streptomycin sulphate 5 mg L-1. The plates were incubated at 24°C for 4 days, and single tips of fungal mycelium were transferred to sterile PDA for purification [20]. Several pure cultures of Rhizoctonia solani isolates were identified by light microscopy as described by Sherwood [21]. Rhizoctonia mycelia were stored at 4°C.
2.3. Chitosan
Chitosan, edible level with an average molecular weight of 150 kDa and degree of deacetylation 80%, was purchased from Chitosan Technologies Limited Company, Engels city, Russia.
2.4. Influence of Chitosan Treatment on Mycelial Growth of Rhizoctonia solani In Vitro
The effect of chitosan on mycelial growth was assessed by inoculating mycelial disks (5 mm in diameter) from the edge of a 4-day-old-culture of the fungus on the center of 90-mm Petri dishes containing 20 ml PDA medium amended with chitosan at (0, 0.125, 0.25, 0.5, or 1%). Plates were incubated in the dark at 25 °C and the mycelial growth was determined by measuring the colony diameter when the mycelium reached to the edges of the control plate. Each treatment was replicated using three plates, and the experiment was performed three times.
2.5. Influence of Chitosan Treatment On Lesion Diameters of Tubers Inoculated with Rhizoctonia Solani
Tubers were wounded (3 mm deep and 3 mm wide) at the equator with a sterile dissecting needle and then mycelial plugs of 4-day-old R. solani culture were inoculated into wounded sites with the hyphal side down. After 3 h of inoculation, tubers were treated with different concentrations of chitosan 0, 0.125, 0.25, 0.5, or 1% (w/v) dissolved in 0.5 mol L-1 glacial acetic acid, and the pH of CH solutions was adjusted to 5.5-6.0 with 1M aqueous NaOH. The treated tubers were incubated in plastic boxes (190 mm× 157 mm × 90 mm) with sterile water to maintain high relative humidity and stored at room temperature (20±2°C). The diameter of the lesions was measured after 30 d of inoculation. Each treatment was applied to three replicates of 15 tubers. The experiment was repeated twice.
2.6. Influence of Chitosan Treatment on Defense-Related Enzymes
Three grams of fresh weight were taken from 3-4 mm below the treated sides (Rhizoctonia with chitosan) and untreated ones (Rhizoctonia lacking chitosan) with a stainless-steel cork borer after 0, 1, 2, 3, 4, 5, 6, and 7 d of treatment with 0.5% chitosan and then were ground to a fine powder in liquid N2 and used for extraction of Peroxidase (POD), Polyphenol Oxidase (PPO) and Phenylalanine Ammonia-lyase (PAL), which are three defense-related enzymes in potato tubers. POD activity was assayed as described by Venisse et al. [22] by measuring the absorbance at 470 nm. PPO activity was estimated according to Jiang et al. [23] by measuring the absorbance at 420 nm. PAL activity was assayed according to the method of Assis et al. [24] by measuring the absorbance at 290 nm. The content of total protein was determined by the method of Bradford [25] using bovine serum albumin as standard.
2.7. Statistical Analysis
All statistical analyses were performed using the CoStat 6.45 software program. To test the effect of chitosan treatment, the data were Analyzed by One-way Analysis of Variance (ANOVA). Mean separations were performed by the least significant difference (LSD) test. Significance was defined as P< 0.05.
3. RESULTS
3.1. Influence of Chitosan Treatment on Mycelial Growth of Rhizoctonia solani In Vitro
Chitosan significantly inhibited the growth of R. solani mycelium in a concentration-dependent manner. The mycelial growth was completely inhibited by chitosan at 1% (Fig. 1).
3.2. Influence of Chitosan Treatment On Lesion Diameters of Tubers Inoculated with Rhizoctonia Solani
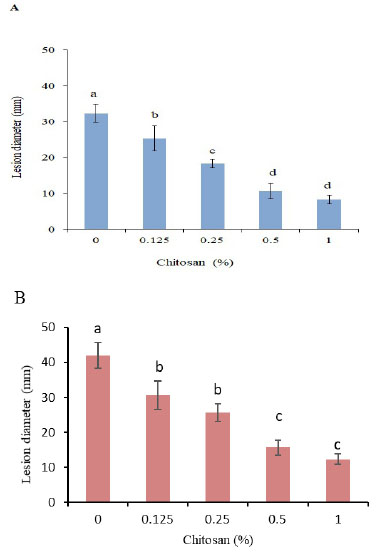
Treatment with chitosan significantly reduced the lesion diameter of R. solani AG-3 PT inoculated tubers after 30 d of incubation and the reduction enhanced with increasing the concentration. However, no significance was found between 0.5 and 1% concentrations in both cultivars (Fig. 2A, B). The CH treatment at 0.5% reduced the lesion diameter by 67.1 and 62.8%, respectively, in Kolobok and Cante cultivars.

3.3. Influence of Chitosan Treatment on Defense-Related Enzymes
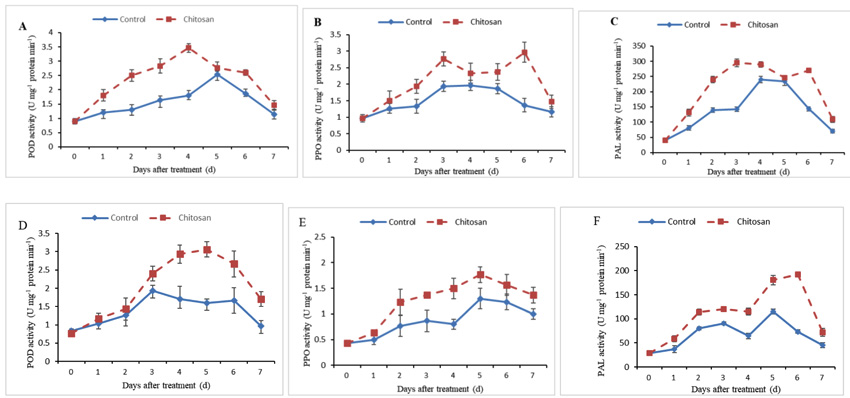
POD was activated following treatment with CH 0.5% in both cultivars Kolobok and Cante. In Kolobok cultivar, a significant increase in POD activity was early observed after 1 day. This response was followed by a strong increase in the activity up to the 4th day (Fig. 3A). In contrast, this response was weaker and delayed in Cante cultivar, the first peak of POD activity appeared only after 3 days of treatment (Fig. 3D). The induction in POD activity reached its higher increase 4 and 5 days after treatment in Kolobok and Cante cultivars, respectively (Fig. 3A, D).
PPO activity pattern was higher in the treated tubers than the control in both cultivars. In Kolobok cultivar, PPO activity showed a different pattern between CH treated and non-treated tubers. Peaks were observed at 3 and 6 days in treated tubers (Fig. 3B). In Cante cultivar, the PPO activity followed the same trend and fluctuation in both CH-treated and untreated control tubers. However, the PPO activity showed a higher induction in CH-treated samples (range of 1.3-1.9 fold increase) than that in the untreated control samples (Fig. 3E).
PAL activity increased with incubation time in CH-treated tubers of both cultivars, reaching maximum values on 3 and 6 days in Kolobok and Cante cultivars, respectively (Fig. 3C, F).
4. DISCUSSION
As a natural elicitor and antifungal agent, chitosan is a promising alternative for the management of postharvest plant diseases [26]. In our present study, chitosan was effective in inhibition R. solani mycelial growth. These observations confirmed similar data on the antifungal effect of chitosan on mycelial growth of R. solani [27, 28], and other several phytopathogenic fungi, such as Fusarium solani [29], Colletotrichum sp [30]. This recent study also showed that chitosan can effectively manage black scurf in potato tubers, by induction of defense-related enzymes such as POD, PPO. These results support the findings of CH-enhanced activities of POD and PPO against Sclerotinia sclerotiorum in carrots [31], Fusarium sulphureum in potato [32], and Alternaria kikuchiana and Physalospora piricola in pear [33]. POD participates in the cell wall building processes, for instance, suberization, phenols oxidation, and lignification of host plant cells during the defense reaction against pathogenic agents [34]. PPO is involved in the oxidation of polyphenols into quinones (antimicrobial compounds) and cells lignification in the infected plants [35]. In this experiment, the activity of PAL has been increased in CH-treated tubers. PAL is the first enzyme in the phenylpropanoid pathway and is involved in the syntheses of phytoalexins, phenols, and lignin which have defense functions in the host plants [36].
CONCLUSION
This study indicated that chitosan directly inhibited the R. solani growth, and potentially elicited defense reaction in potato tubers.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the Biotechnology Lab., Agronomy Faculty, Saratov State Vavilov Agrarian University for the support and technical assistance of this research.


