All published articles of this journal are available on ScienceDirect.
Impacts of Microbial Inoculants on the Growth and Yield of Maize Plant
Abstract
Background:
The use of microbial inoculants holds a great promise to improve crop yield without the negative environmental and health hazard associated with chemical fertilizer.
Aim:
To investigate if Pseudomonas spp. (Pseudomonas kilonensis F113 and Pseudomonas protegens CHA0 strains) have promoting effects on vegetative growth and yield of different maize genotypes (viz. AFLATOXIN SYN 4W, TZB-SR, AFLATOXIN R SYN 2Y, AFLATOXIN SYN 3W and AFLATOXIN SYN-2Y) under different soil types.
Methods:
Both pot and field experiments were employed. Bacterialized seeds were sown (2 seeds/pot/stand).
Results:
Pot experiment showed that both the bacterial species significantly stimulated the growth of maize shoot length, stem girth, leaf length, root length and root weight. The effect of genotypes AFLATOXIN SYN 4W, TZB-SR, AFLATOXIN R SYN 2Y and AFLATOXIN SYN 3W are not significantly different from one another but AFLATOXIN SYN-2Y showed a significantly lower increase in the measured parameters. No significant difference was observed according to soil types. AFLATOXIN SYN 4W showed a significantly higher root weight while AFLATOXIN R SYN 2Y showed a significantly higher root length compared to the other maize genotypes. Moreover, Pseudomonas significantly increased maize growth and yield under field experiment. AFLATOXIN R SYN 2Y and AFLATOXIN SYN 4W showed a significantly higher yield than the other maize genotypes studied.
Conclusion:
We concluded that Pseudomonas kilogenensis F113 and Pseudomonas protegens CHA0 are potential biofertilizers.
1. INTRODUCTION
Maize (Zea mays L.) is a common cereal that is intensively cultivated worldwide [1]. Maize genotypes differ in starch structures and composition of maize kernels (content of amylase and/or amylopectin), grain filling rate, type of endosperm, i.e. floury (dent) vs. horny (flint) and in earliness and rate of maturation [2]. Maize genotypes can also differ in a number of plant growth characters [3] or drought stress resistance ability [4].
Numerous agricultural soils worldwide are deficient in plant nutrients. Hence, significant fertilizer requirement is a major challenge for sustainable food production. Previously, these plant nutrients were provided solely in the form of synthetic chemical fertilizers. Such chemical fertilizers are quite expensive and increase crop production cost. In addition, chemical fertilizers lead to soil degradation and pose health hazard to both human and farm animals [5]. It is therefore imperative to improve soil fertility, while at the same time preventing associated negative environmental effects of chemical fertilizers.
Microbial inoculants are of growing interest for their potential role in improving soil fertility and enhancing an increase in crop yields and their nutrient contents. Microbial inoculants are the formulations composed of beneficial microorganisms that play important role in every ecosystem. When applied to seeds, soil or seedlings, microbial inoculants improve directly or indirectly the nutrient availability to the host plant and promote plant growth [6, 7]. They hold a great promise to improve crop yield (Isfahani and Besharati 2012). In the present agricultural practices, there is a number of beneficiary soil microorganisms used as inoculants. They include Pseudomonassp, Azospirillum, Azotobacter and Phosphobacterium among others [8, 9]. Microbial inoculants improve plant growth through a number of mechanisms which include the production of plant hormones, the supply of nutrients and the suppression of various crop pests [10].
Pseudomonas is a predominant group of rhizosphere soil colonizing bacteria, which have been reported to promote plant growth [11]. Pseudomonas spp. rhizosphere strains have been described as effective phosphate solubilizers [12]. Verma et al. [13], noted that Pseudomonas putida strain BHUPSB04 solubilized tricalcium phosphate in soluble form by producing organic acid. Likewise, co-inoculation of Galega orientalis Lam. with root-colonizing Pseudomonas species (Pseudomonas trivialis strain 3Re27 and Pseudomonas extremorientalis strain TSAU20) and Rhizobium, resulted in enhanced root and shoot mass [14]. However, there have been very little reports on the impact of plant genotypes and soil types on the growth promoting potential of Pseudomonas spp.
In this investigation, we therefore aimed to determine if Pseudomonas spp. (Pseudomonas kilonensis and Pseudomonas protegens strains) have promoting effects on vegetative growth and yield of different maize genotypes under different soil types through the analysis of some indicating factors, such as root length, plant height, root weight and yield of maize plants.
2. MATERIALS AND METHODS
2.1. Plant Material
Seeds of maize cultivars (Aflatoxin SYN 4W, TZB-SR, Aflatoxin R SYN-2Y, Aflatoxin SYN 3W and Aflatoxin SYN-2Y) used in this investigation were obtained from the International Institute of Tropical Agriculture, Ibadan, Nigeria. The seeds were sterilized with 70% ethanol for 2 minutes and washed with sterile distilled water.
2.2. Microbial Inoculant Preparation
P. kilonensis and P. protegens isolates selected for this study were obtained from the Microbial Ecology laboratory (C. Prigent-Combaret). The bacterial isolates were cultured for 48 hrs in Luria-Bertani Broth (LB) medium, and then cells were harvested by centrifugation at 12000g for 10 minutes. Each of the P.kilonensis and P. protegens was suspended in sterile distilled water and the concentration adjusted to give 108cells/ml [15].
Ten grams of pectin were suspended in 1 L each of P. kilonensis and P. protegens suspensions, which were shaken for 10 min on a magnetic stirrer plate. Maize seeds were imbibed at the ratio of 500 g/L microbial solutions for 16 hrs.
The bio-inoculated seeds were then air-dried on filter paper for 1 hr before sowing. Another group of surface-sterilized maize seeds (70% ethanol for 2 min) were prepared as control treatments [16].
2.3. Collection of Soil Samples
Soil samples were collected randomly from Mafikeng and Itsoseng areas in South Africa. Mafikeng is located at 25°50'S, 25°38'E. The soil was grey loamy sand with 84% sand, 3% clay and 13% classified as Hutton and Itsoseng is located at 26°08'S, 25°86'E [17]. The soil was red sandy loam with 6.5% silt, 9.8% clay 83.6% sand classified as Ferallic Arenosols [17]. Using the random sampling method, auger samples (5kg) were collected from each sampling units at 0-15 cm. The soil samples collected from each of the sampling sites were bulked and transported to the laboratory in well labelled polyethylene bags. The core samples were then air dried for 3 days and passed through 2 mm sieve in the preparation for analysis.
2.4. Determination of Physical and Chemical Properties of Soil of Study Sites.
The physical and chemical properties measured include: pH using Kent pH metre model 7020, organic matter content using the wet oxidation method as described by [18]. The hydrometer method of Gee and Or. was employed in the determination of particle size [19]. Total nitrogen was estimated by the macro Kjeldahl method [20], available phosphorus (P) was determined by Bray-1 extraction method [21]. To determine E.C.E.C. the method of Chapman was employed [22]. These are shown in Table 1.
| Physical and Chemical Properties of Soils | Itsoseng Soil | Mafikeng Soil |
|---|---|---|
| pH (H2O | 6.44± 0.11 | 6.81± 0.03 |
| OM (%) | 0.79± 0.03 | 1.66± 0.06 |
| P (mg/kg) | 5.72± 0.04 | 1.39±0.04 |
| N (%) | 0.06± 0.01 | 0.11±0.02 |
| Na+ (cmol(+)/kg) | 2.80± 0.03 | 2.60±0.02 |
| K+ (cmol(+)/kg) | 3.25± 0.03 | 3.25±0.07 |
| Ca2+ (cmol(+)/kg) | 2.60± 0.02 | 2.55± 0.02 |
| Mg+ (cmol(+)/kg) | 1.20± 0.01 | 0.20±0.02 |
| Exchangeable acidity (cmol(+)/kg) | 3.68± 0 .08 | 2.24±0.02 |
| ECEC (cmol(+)/kg) | 13.53± 0.1 | 10.84± 0.03 |
| % Silt | 6.5± 0.03 | 13.0±0.27 |
| % Clay | 9.88± 0.04 | 2.88± 0.02 |
| % Sand | 83.62± 0.04 | 84.12±0.03 |
| Class | Sandy Loam | Loamy Sand |
| Genotype | Grain Colour | Grain Size | Type |
|---|---|---|---|
| Aflatoxin Syn-2Y | Yellow | Small | Flint |
| Aflatoxin SYN 4W | White | Very small | Flint |
| TZB-SR | White | Small | Flint |
| Aflatoxin R Syn-2Y | Yellow | Small | Flint |
| Aflatoxin SYN 3W | White | Small | Flint |
2.5. Plant Growth Promotion Studies Under Screen House
A pot experiment was laid in a Randomized Complete Block Design (RCBD) split plot arrangement. The main plot was Pseudomonas spp. (P. kilonensis, P. protegens CHAO and non-inoculated control) applied as seed treatment while the sub plots were maize genotypes (AFLATOXIN SYN 4W, TZB-SR, AFLATOXIN R SYN 2Y, AFLATOXIN SYN 3W and AFLATOXIN SYN-2Y 4W, TZB, AR, 3Wand 2Y) and 2 soil types (Itsoseng soil and Mafikeng soil). Bacterialized seeds were sown (2 seeds/pot) in 35cm-diameter pots filled with 12 Kg of sterilized soil. Soil was wet sterilized in an autoclave at 121ºC for 20 minutes. This is to ensure that only the inoculant introduced influence the sown seed. Only one plant per pot was maintained after germination. All treatments were in triplicates. Plants were irrigated daily at 6.00 hr and 18.00 hr using a watering can. Neither inorganic fertilizer nor pesticide was applied. Physical and chemical properties of the studied soils are shown in Table 1. While the phenotype characteristics of seeds used are recorded in Table 2.
2.6. Growth Parameter Data Collection
Growth parameters (plant height (cm) using a measuring tape, leaf length (cm) using a measuring tape, plant girth (cm) using a thin thread whose length was measured on a ruler, root length (cm) i.e length of the longest root using a measuring tape and root dry weight (g) per plant) were recorded at 12 weeks after planting. To obtain the root dry weight, the below ground portion of the plant was cut and washed to remove attached soil particles. The root was then air dried to a constant weight and the weight was recorded.
2.7. Assessing Plant Growth Promotion of Pseudomonas Spp. Under Field Condition
A field experiment was conducted between September, 2015 and February 2016 at North-West University, Mafikeng, (25°50'S, 25°38'E) South Africa. The Pseudomonas spp. tested were P kilonensis F113 and P protegens CHA0. Maize genotypes used include Aflatoxin SYN 4W, TZB-SR, Aflatoxin R SYN 2Y, Aflatoxin SYN 3W and Aflatoxin SYN-2Y.The soil of the study site was a loamy sand soil, slightly acidic with organic matter value of 1.66%, available phosphorus 1.39PPM, total nitrogen was 0.11 and effective cation exchange capacity was 10.84 (Table 1).
2.8. Experimental Layout
The experimental design was a randomized complete block (RCBD) split plot arrangement with three replications. The main plot was Pseudomonas spp. (P. kilonensisF113, P. protegens CHA0 and non-inoculated control) applied as seed treatment, while the sub plots were maize genotypes (Aflatoxinsyn 4w, TZB-SR, Aflatoxin R SYN 2y, Aflatoxin SYN 3W and Aflatoxin syn-2y). Plot size was 2.2 m2. Interplant and row distance were 50 cm X 50 cm respectively. This gave a plant population of 8 plants per plot which is equivalent to 40,000 plants per hectare. Maize was sown at 3 seeds per hole. This was thinned to two per stand after germination and allowed to grow to maturity. Irrigation was solely by rainfall. Mafikeng had an average annual rainfall of 300 to 700 mm and temperatures range between 22ºC and 34ºC. No fertilizer was applied. Weeding was carried out manually using hoe at 4weeks, 8 weeks and 12 weeks after planting. Pest was controlled by the microbial inoculant. No chemical pesticide was applied. The maize varieties matured at different date however harvesting was carried out same day as maize were left on the stalk in the field to dry.
2.9. Growth Parameter and Yield Data Collection
Growth parameters (plant heights (cm) using a measuring tape, leaf length (cm) using a measuring tape, stem girth (cm) using a thin thread whose length was measured on a ruler were recorded at maturity. Dry maize cobs from each treatment were harvested threshed and the threshed maize seeds were weighed on a weighing scale and recorded as yield (tonnes/ha).
2.10. Statistical Analysis
Data collected from field and pot experiments were subjected to two way Analysis of Variance (ANOVA) using IBM SPSS statistical package 21. Means were compared using Duncan Multiple Range Test (p<0.05).
| Variables | Plant Height (cm) | Leaf Length (cm) | Plant Girth (cm) |
|---|---|---|---|
| Soil Type | |||
| Itsoseng | 95.01a | 73.22a | 6.11a |
| Mafikeng | 95.47a | 73.53a | 6.23a |
| Inoculants | |||
| Pseudomonas kilonensis F113 | 99.38a | 74.60a | 6.48a |
| Pseudomonas protegensCHA0 | 96.36b | 71.09b | 6.01b |
| Non inoculated | 90.29c | 74.60a | 6.09b |
| Maize Genotype | |||
| Aflatoxin SYN 4W | 95.37a | 74.04a | 6.29a |
| TZB SR | 96.67a | 74.40a | 6.17ab |
| Aflatoxin R SYN 2Y | 96.41a | 74.03b | 6.19ab |
| Aflatoxin SYN 3W | 97.52a | 74.74a | 6.28a |
| Aflatoxin SYN -2Y | 90.74b | 69.93b | 6.04b |
| Treatment Effects | |||
| Soil Type | 0.958 | 0.948 | 0.064 |
| Inoculant | 0.000 | 0.000 | 0.000 |
| Maize genotype | 0.006 | 0.005 | 0.011 |
| Soil Type × Inoculant | 0.224 | 0.955 | 0.319 |
| Soil type × maize genotype | 0.001 | 0.008 | 0.000 |
| Inoculant × maize genotype | 0.000 | 0.000 | 0.000 |
| Soil type × Inoculant ×maizegenotype | 0.502 | 0.508 | 0.000 |
3. RESULTS
3.1. Effects of Microbial Inoculants, Soil Types and Maize Genotype on the Growth of Maize Planted in Pots
The results in Table 3 show that plant height, leaf length and plant girth on Itsoseng soil were not significantly different from those on Mafikeng soil. Both P. kilonensisF113 and P. protegens CHAO significantly increased the plant height. P. kilonensis F113 significantly increased plant girth. However, P. protegens CHAO decreased leaf length significantly. Also the growth parameters measurement significantly differed according to maize genotypes (P<0.05).
Combined effect of soil type × maize genotype and microbial inoculant × maize genotype increased significantly the plant height, leaf length and plant girth, while soil type × inoculant × maize genotype significantly increased the plant girth. However, combined effect of soil type × microbial inoculant was not significant on plant height, leaf length and plant girth as reported in Table 3.
Means followed by the same letter along the column for a factor are not significantly different at 5% level of probability using Duncan Multiple Range Test (DMRT).
3.2. Effects of Microbial Inoculants, Soil Types and Maize Genotype on Root Weight and Root Length of Maize Planted in Pots.
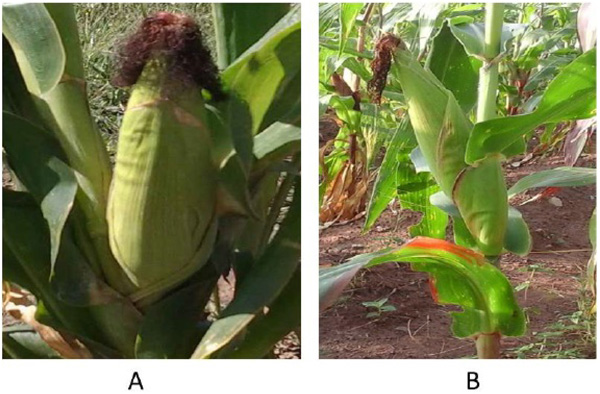
The effects of microbial inoculants, maize genotypes and soil type on root weight and root length of maize planted in pot was shown in Table 4. Maize plants inoculated with P. kilonensis F113 significantly increased root weight and root length. Soil from Mafikeng produced a significantly heavier and longer root system than soil from Blaawbank. Maize genotype Aflatoxin SYN 4W produced a significantly higher root weight while maize genotype Aflatoxin R SYN 2Y had a significantly higher root length compared to the other genotypes. Interactions of inoculant × maize genotype, and soil type × inoculant × maize genotypes had significant effect on the both root weight and root length of maize while soil type × inoculant and soil type × maize genotype had significant effect on root length but not on the root weight. Microbial inoculation thus resulted into elongation of stems and roots (Fig. 1).
3.3. Effects of Microbial Inoculants on the Growth and Yield of Maize Planted on the Field
Table 5 shows the effects of Pseudomonas inoculants on the growth and yield of maize planted on the field. Microbial inoculants had very high significant improved effect on growth and yield performance of maize plant. Both P. kilonensis F113 and P. protegens CHA0 significantly increased the plant height, leaf length and the yield of maize (Fig. 2 and Table 5). However, plants inoculated with P. kilonensis F113 had a higher height than those inoculated with P. protegens CHA0. This experiment evidence that the maize genotypes were not significantly different in growth but Aflatoxin SYN 4W and Aflatoxin SYN -2Y produced significantly higher yield followed by TZB SR and Aflatoxin SYN 3W while Aflatoxin R SYN -2Y has the least yield. There was no significant difference between maize genotypes for both growth and yield of maize. Similarly, the effect of maize genotype × inoculant on the growth and yield of maize is not significant. The growth and yield of all the genotypes tested were increased by the inoculants (P=0.05).

| Variables | Root Weight (g) | Root Length (cm) | |
|---|---|---|---|
| Soil Type | |||
| Itsoseng | 8.08b | 46.16b | |
| Mafikeng | 17.65a | 50.16a | |
| Inoculants | |||
| Pseudomonas kilonensisF113 | 20.20a | 53.93a | |
| Pseudomonas protegens CHA0 | 9.90b | 45.53b | |
| Non inoculated | 13.28b | 47.00b | |
| Maize Genotype | |||
| Aflatoxin SYN 4W | 23.71a | 46.67b | |
| TZB SR | 10.77b | 46.19b | |
| Aflatoxin R SYN 2Y | 14.21b | 53.26a | |
| Aflatoxin SYN 3W | 10.41b | 48.19b | |
| Aflatoxin SYN 2Y | 13.19b | 49.81ab | |
| Treatment Effects | |||
| Soil Type | .000 | .014 | |
| Inoculant | .000 | .000 | |
| Maize genotype | .000 | .004 | |
| Soil Type × Inoculant | .000 | .203 | |
| Soil type × maize genotype | .000 | .436 | |
| Inoculant × maize genotype | .000 | .000 | |
| Soil type × Inoculant × maizegenotype | .000 | .013 | |
4. DISCUSSION
The results of this research agree with some other studies where microbial inoculation improved plant growth. Application of microbial inoculants as revealed by Akladious and Abbas caused increase in all measured growth parameters of maize plant [23]. Inoculation of maize seeds with P. putida strain R-168, P. fluorescens strain R-93, P. fluorescens DSM 50090, P. putida DSM291 significantly increased plant height in a field experiment [24]. The growth and yield of maize DMRESRY and EV99-MRP were differentially influenced by the application of bio-fertilizer P. fluorescens [25]. According to Oufdou et al. the plant genotype could also influence the phosphorus uptake released by phosphorus solubilizing bacteria from insoluble phosphate [26]. P. fluorescens has also shown ability to adapt to survival in soil and colonize the roots of plants [27] and to protect plant against drought stress [4]. Pseudomonas protegens was reported to produce biocontrol compounds [28]. Likewise Stockwell et al. and Yasmin et al. reported biocontrol potential of Pseudomonas spp., against a host of plant disease organisms [29,30].
Isfahani and Besharati also reported an increase in root weight of cucumber inoculated with Pseudomonas sp. soil type, microbial inoculant, maize genotype have significant positive effect on both root weight and root length of maize [31].
Table 4 confirms the report by Babalola that microbial activity in the rhizosphere for nutrient acquisition affects root morphology and/or physiology [32]. The stimulation of root growth can enhance plant nutrient uptake [33]. Microbial inoculants often increase plant growth by promoting root development and alter root architecture via the production of phytohormones like Indole-3-Acetic Acid (IAA). This results in increased root length, root surface area numbers of root tips and volume [34,35]. Such stimulation of roots can aid plant defence against pathogens and can also relate to Induce Systemic Tolerance (IST) [36]. Since sites of nutrient uptake include root surfaces and root tips, it is likely that one mechanism by which microbial inoculants lead to increased nutrient uptake is via stimulation of root development [37]. A study to investigate effect of P. fluorescens on plant growth promotion under in vitro and in situ conditions conducted by Katiyar and Goel revealed that P. fluorescens produce a significant increase in root (30 and 20%) and shoot length (20 and 24%) of mung bean (Vigna radiata) via phosphorus solubilisation by acid production which results in a decrease in pH [38].
Babalola and Glick reported increased plant growth as one of the benefits of microbial inoculants [39]. A similar result was reported by Botelho [40]. They showed that Pseudomonas fluorescens strain BR-5 stimulated the growth of maize in a natural soil. Microbial inoculants improve plant growth and increase productivity as well as the host plant nutrient status [33].
| Variables | Yield (t/ha) | Plant Height (cm) | Leaf Length (cm) |
|---|---|---|---|
| Inoculants | |||
| Pseudomonas kilonensis F113 | 555a | 111.27a | 68.38a |
| Pseudomonas protegensCHA0 | 555a | 104.49a | 68.38a |
| Non inoculated | 122b | 41.27b | 32.40b |
| Maize genotype | |||
| Aflatoxin SYN 4W | 473a | 90.93a | 61.16a |
| TZB SR | 416ab | 87.89a | 56.00a |
| Aflatoxin R SYN 2Y | 309b | 76.66a | 57.18a |
| Aflatoxin SYN 3W | 362ab | 77.51a | 50.83a |
| Aflatoxin SYN -2Y | 498a | 95.39a | 57.18a |
| Treatment Effects | |||
| Inoculant | 0.000 | 0.000 | 0.000 |
| Maize genotype | 0.90 | 0.489 | 0.538 |
| Inoculant × maize genotype | 0.34 | 0.521 | 0.435 |
Significant difference in plant growth and yield were observed among inoculated and un-inoculated treatments in all the maize genotypes tested. Pseudomonas kilonensis F113 and Pseudomonas protegens CHA0 increased maize plant height, plant girth, and leaf length in both screen house and field experiments with Pseudomonas kilonensis F113 performing the best. The root length and root weight were also enhanced by these organisms in the screen house experiment. Pseudomonas kilonensis F113 also produced the highest yield under field experiment. The impact of microbial inoculants on maize growth and yield were influenced by soil type and crop genotype. Aflatoxin SYN 4W had the best performance. Pseudomonas kilonensis F113 and Pseudomonas protegens CHA0 are potential biofertilizers for maize plants.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
North-West University is gratefully acknowledged for ETA post- doctoral supports. OOB would like to thank the National Research Foundation, South Africa for grant (Ref: UID81192) that have supported research in her laboratory.
Landmark University Omu-Aran, Nigeria is equally ack-nowledged for financial support to get this article published.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Decalred none.


