All published articles of this journal are available on ScienceDirect.
Curative Activity of Watery Fermented Compost Extract as a Bark Treatment against Tapping Panel Dryness
Abstract
Background:
Tapping panel dryness (TPD) is a stress-related disorder that afflicts rubber trees, contributing to yield losses in nearly every rubber-growing region.
Method:
We demonstrated the curative effects of biostimulants containing a fermented watery extract of shrimp waste-enriched compost (SWCE) on TPD in field trials. Undiluted SWCE was applied to lightly scraped bark in the first, third, and fourth trials, and applied directly without bark scraping in the second trial.
Results:
Bark treatment significantly (p < 0.05) reduced tapping cut dryness and increased latex yield, suggesting recovery from the disorder. When SWCE was applied to pre-scraped bark, 80% and 30% of trees with partial and complete TPD, respectively, recovered from tapping dryness within 2 months. The latex dry weight of treated trees with partial and complete TPD was 77.5% and 21.1% that of healthy trees, respectively. We observed slight recovery from TPD in trees treated without bark scraping and in trees with a history of ethephon stimulation. No curative effect of SWCE was demonstrated in treated trees without a tapping rest period. These findings suggest that compost extract could be a useful treatment for partial TPD.
1. INTRODUCTION
Tapping panel dryness (TPD) is a physiological disorder afflicting rubber trees resulting from stresses related to excessive recurrent tapping and overstimulation by ethylene [1-4]. The disorder causes severe yield and crop losses in natural rubber-producing countries [5]. TPD is detected early by bark dryness upon tapping, which can manifest as partial dry zones (no latex flow) [6]. Ultimately, the disease causes a complete stoppage of latex flow on the tapping cut [7]. The early onset of the syndrome is tapping cut dryness, which lacks any visible sign of bark necrosis and is related to overproduction of reactive oxygen species (ROS) in laticifers [3]. This type of TPD is reversible after a resting period for the trees [8]. In the advanced stage, an irreversible type of total dryness, called bark necrosis [9] or brown bast TPD (BB-TPD), can occur [3]. The latter, which is related to a cyanogenesis process [7, 10], involves histological deformation of the bark including browning, thickening, or even flaking due to thylosoid formation, lignified gum, and abnormal division of parenchyma cells [3, 9].
A great deal of research has been done to reveal the nature and molecular mechanisms of TPD. However, data are lacking on the bioactive compounds for recovery from the disorder. In reversible TPD, affected trees can sometimes be cured by bark scraping and application of chemicals. Tapping can be reconsidered after a resting period for bark regeneration. However, this process is costly, and a year of latex production can be lost [3]. TPD is a stress-related disorder, and the bioactive compounds and/or microorganisms that can enhance stress tolerance are being developed as agents for the curative treatment of the disorder. Plant growth stimulation and enhanced tolerance to biotic and abiotic stresses have been reported following the application of a variety of bioactive compounds, including humic and amino acids, peptides, saponins, alginates, mannitol, and fatty acids [11].
The application of compost water extract (CWE), popularly known as compost tea, is a simple and inexpensive method to extract plant beneficial bioactive compounds from compost into the solution [12]. Improved plant growth, yield, and nutritive quality as well as disease suppression in response to CWE foliar spray or soil drench, have been reported elsewhere [13-19]. This study examined the suppression of stress-related disease through bark treatment with a CWE from shrimp shell-enriched compost.
2. MATERIALS AND METHODS
2.1. Watery Fermented Compost Extract
Shrimp waste-enriched compost extract (SWCE) was produced from shrimp waste-enriched compost through two-step fermentation. The enriched compost was fermented by suspension in water and then left undisturbed at ambient temperature for 4 days to extract the bioactive substances. The supernatant was filter-harvested and mixed with 5% (w/v) sucrose and 10% (v/v) compost activator. The entire brewer contents were vigorously stirred by hand and then left to ferment at ambient temperature for 21 days. SWCE can be stored (without significant changes in nutrient contents) in a closed plastic container for 5 years [20]. Its plant nutrients are composed of mainly nitrate (350 ppm), calcium (450 ppm), as well as amino acids including glycine (365 ppm), aspartic acid (232 ppm), lysine (184 ppm), leucine (186 ppm), glutamic acid (170 ppm), and valine (132 ppm).
2.2. Trials with Tapping Rest and No Ethephon Stimulation
Trials involved bark treatment firstly with bark scraping and secondly, without bark scraping. Both trials were performed at the Faculty of Agriculture, Sriwijaya University Experiment Station, Gelumbang, South Sumatra. The plantation was established in 1999 with a GT1 clone and tapped using a system of 1/2S d/2 (a half spiral cut alternating daily). Ethephon stimulation was not applied at this plantation.
We applied 30 ml undiluted SWCE using a brush on recently scraped bark (panel BO-1 or BO-2) in the first trial and directly without prior bark scraping in the second trial. Bark scraping consisted of the removal of the outer layers of cork to 30 cm below and above the tapping cut. In total, 60 trees were treated in the first trial, and another 80 trees were used in the second trial. Half of the treated trees had no latex flow on the tapping cut (total TPD), and in the remainder, the cut length was 45-65% dry (partial TPD). All TPD trees were without brown color or necrosis on the bark. Trees were treated once (single application), treated twice at a 1-month interval (double application), or brushed with water (control) in the first trial. The second trial included four treatments (SWCE, SWCE + 5% KCl, SWCE + 5% NaCl, and water as control). Each treatment was applied twice (with a 1-month interval), and each treatment had 10 replicates. Treated trees were not tapped during the trials.
2.3. Trials with Ethephon Stimulation and Tapping Rest
The third trial was conducted on 10-year-old rubber tree clones (PB260) at a commercial rubber plantation in Ogan Ilir, South Sumatra. Trees were tapped using a system of 1/2S d/3 and stimulated monthly with 2.5% ethephon. The trial included bark treatment with SWCE on scraped bark in total- or partial-TPD trees. Treatment was applied three times at a 2-month interval. Water was applied to the control trees. There were 15 replicates. Treated trees were not tapped during the experiment.
2.4. Trials with Ethephon Stimulation and without Tapping Rest
The fourth trial was conducted on 13-year-old rubber tree clones (PB260) at a small-holding rubber plantation in Gelumbang, South Sumatra. The trees in this trial were overexploited by daily tapping (1/2S d/1) and stimulated monthly with 2.5% ethephon. SWCE was applied three times at a 1-month interval on the scraped bark of partial-TPD trees. The treated trees were tapped daily without a rest during the experimental period.
2.5. TPD Recovery
The trees were tapped three times at a cutting interval of 2 days (1/2S d/3) at the following times after first application: first trial: 2 months; second trial: 1 and 2 months; third trial: 5, 7, and 10 months; fourth trial: 2, 3, and 4 months. Tapping cut dryness was measured as a percentage of dry cut length relative to the total length of the tapping cut and was observed immediately after tapping. The latex yield was measured as the latex volume and dry weight [21]. To study the effect of SWCE on the plugging index, the latex flow rate for the first 5-minute tapping was measured and divided by the total volume [22].
The results were examined using analysis variance and the Waller-Duncan K-ratio t-test (p = 0.05) using the agricolae and Rcmdr packages in the R statistical software (version 3.3.1; R Foundation for Statistical Computing, Vienna).
3. RESULT
3.1. Trial with Tapping Rest and without Ethephon Application
We consistently observed a reduction in tapping cut dryness and an increase in latex yield in trees with both total and partial TPD in response to bark treatment, indicating recovery from the disorder. Higher latex stimulation was observed in TPD trees with bark scraping and double SWCE application (Fig. 1).
In trials with bark scraping, 8 of the 10 treated partial-TPD trees and 3 of the 10 total-TPD trees recovered from tapping cut dryness. On partial- and total-TPD trees treated with SWCE, the percentage of dry cut length was significantly (p < 0.05) lower than that in the control (Fig. 2). The treatment resulted in a reduction of the dry cut by 69.1% and 91.4% relative to control following single and double applications of SWCE to partial-TPD trees, respectively. When SWCE was applied to total-TPD trees, dry cut was reduced by 69.6% and 82.7% relative to control following single and double applications.
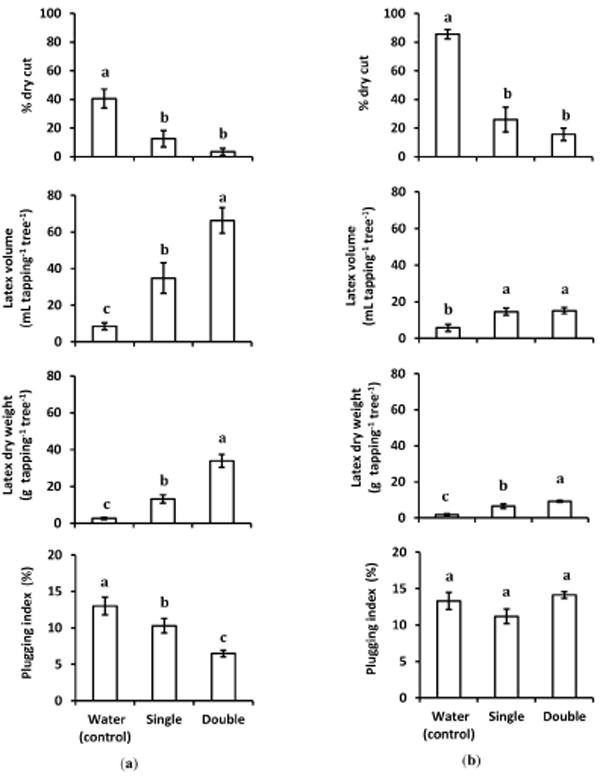
The latex yield (i.e., latex volume and dry weight) of treated partial-TPD trees was significantly increased (p < 0.05) after SWCE treatment, and this increase was larger following double application (Fig. 2). The latex dry weight of treated partial TPD increased 11.8 fold relative to control, the equivalent of 77.5% of healthy trees (average: 43.7 g tapping-1). The tapping cuts of treated total TPD started to produce latex with dry weights that were 21.1% those of healthy trees.
The plugging index was significantly (p < 0.05) reduced with an increase in recovered latex yield in partial-TPD trees. However, no reduction in plugging index was observed in treated total-TPD trees (Fig. 2). The tapping cuts of treated total-TPD trees started to secrete latex, but this latex immediately coagulated in laticifers within 5-10 minutes. Bark scraping alone could induce latex secretion, as observed in water-treated total-TPD trees that started to produce small amounts of latex (Fig. 2), whereas no latex was secreted in trees without bark scraping (Fig. 3).
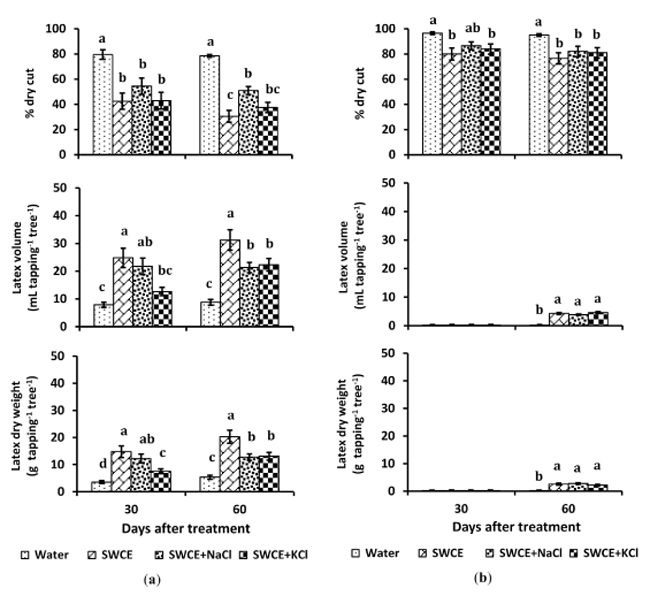
When SWCE was directly applied without bark scraping (second trial), the percentage of dry cut length of the treated tapping panel in both partial- and total-TPD trees was significantly lower (p < 0.05) than that of the control. The latex yield of treated TPD trees was significantly higher (p < 0.05) than that of the control (Fig. 3). However, when compared to trees treated with bark scraping, treatments without bark scraping resulted in a smaller reduction in tapping cut dryness and reduced stimulation of latex yield. The dry cut of the treated partial- and total-TPD trees decreased by 61.1 and 19.5% relative to control, respectively. The latex dry weight of treated partial-TPD trees increased 2.8 fold relative to control, or 56.8% of healthy trees (average: 43.7 g tapping-1). In treated total-TPD trees, tapping cuts produced small amounts of latex, equal to 5.8% of the latex dry weight of healthy trees.
A more substantial recovery effect due to bark treatment with SWCE was observed 1 month after application. Treatment without bark scraping on partial-TPD trees resulted in a 46.5% decrease in tapping cut dryness after 1 month, and a 61.1% reduction was obtained after 2 months. Latex dry weight increased 3.2 fold relative to control after a 1-month application. The addition of 5% (v/v) KCl or NaCl salt to the SWCE significantly reduced (p < 0.05) the biostimulant activity of the mixture. Even though partial TPD trees treated with the salted SWCE produced higher latex yields relative to the controls, the yields were lower than those of non-salted SWCE (Fig. 3).
3.2. Trials in Trees with Ethephon Stimulation and Tapping Rest
No bark-treated trees exhibited total recovery from TPD in this trial, but their tapping cut dryness decreased and latex volume increased in response to the treatment. The percentage of the dry cut length of treated partial-TPD trees was significantly (p < 0.05) lower than control and 36-41% less relative to control at the 7th and 10th months. Similar results were observed for total-TPD stress. The percentage of dry cut length was significantly lower in treated trees compared to control and was 23-43% less relative to control than values between the 5th and 10th months. The dry cut length of control TPD trees tended to increase between the 5th and 10th months Fig. (4).

Stimulation of latex yield was observed in treated partial- and total-TPD trees in this trial. The beginning of latex production was observed in 8 of the 15 treated trees (16%), compared to a reduction in latex production in water-treated control trees between 5 and 10 months after application. The latex volume of treated partial-TPD trees was significantly higher (p < 0.05) than that of control, and increased 77-96% relative to control from the 5th to the 10th month. Under total-TPD stress, bark treatment resulted in a 59-95% increase in latex volume relative to control, although a significant difference was observed only at the 7th month (Fig. 4). However, compared to healthy, treated TPD trees, these produced small amounts of latex until 10 months after the first bark treatment. The latex volume in treated partial and total TPD was 17.1% and 6.6% that of the healthy trees (average: 168.1 mL latex tapping-1).
3.3. Trials with Ethephon Stimulation and without Tapping Rest
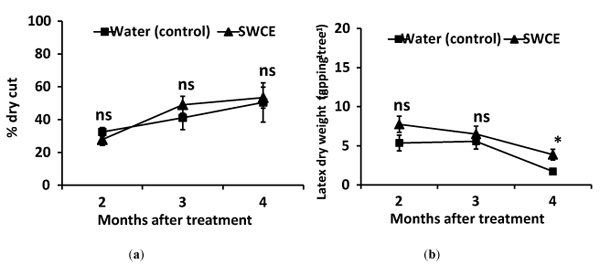
The treated trees were tapped daily without a resting period. No recovery effect was observed after bark treatment with SWCE on these over-exploited rubber trees. The percentage of dry cut length was shown to increase over time on both the treated and control trees. Latex dry weight tended to be higher on treated compared control trees; however, the latex yield was found to decrease with an increase in dry cut length (Fig. 5).
4. DISCUSSION
Bark treatment with SWCE consistently reduced dry cut length and increased latex yield in TPD affected trees. The increase in latex yield was much higher in partial- compared to total-TPD trees, suggesting that bark treatment is more effective during the early stages of the syndrome. Conversely, there was no evidence of self-recovery in water-treated TPD trees during this study. The dry cut length of control trees increased even after a 10-month rest from tapping. Therefore, curative treatment is necessary to suppress syndrome development.
In all trials, bark treatments on total-TPD trees resulted in poor disease recovery compared to those on partial-TPD trees, indicating that the treatment was less effective when applied during advanced stages of TPD when histological deformation of the bark occurred due to thylakoid formation, lignified gum, and abnormal division of parenchyma cells, ultimately causing irreversible total latex dryness [9]. The tapping cut of some treated trees started to secrete latex, but the latex was immediately coagulated (high plugging index), leading to low yield due to the short duration of flow during tapping. This effect was probably due to higher cyanogenesis on the laticifers that resulted in unstable latex [10].
When ethephon was applied frequently, bark treatments with SWCE resulted in a decrease in curative effects compared to those in trees without a history of ethephon stimulation. There was no curative effect from the treatment in over-exploited trees that were tapped daily without a rest during the experimental period. Resting from tapping is necessary for effective curative treatment with SWCE. A high tapping frequency and ethephon stimulation have been known to produce over-accumulation of ROS and to cause oxidative stress that ultimately leads to laticifer dysfunction [3, 23]. The addition of 5% (w/v) KCl or NaCl significantly inhibited the curative action of SWCE. Inhibition of salts under biostimulation activity could be explained by the induction of ROS and ethylene production when a plant is exposed to salt stress [24]. It is likely that the curative effect of SWCE is greatly affected by physiological stress in the individual tree, but the underlining mechanism needs to be further investigated.
Disease suppression, improved plant growth and yield following soil and foliar application of 0.2-2.0% SWCE have been demonstrated in our pot and field trials. The application of compost extract increased yield of ratooned rice crops [25] and suppressed blast disease (S. Suwandi, unpublished data) in a tidal swamp area in South Sumatra. Increased growth of rice seedlings treated with SWCE has been observed under salinity stress [26]. Fast leaf greening (usually within 3 days) and delays in leaf senescence are among common plant responses following application of the extract, an observation similar to well-known cytokinin effects [27]. Krishnakumar et al. [28] reported that cytokinin and trans-zeatin riboside levels were lower in the bark tissue of TPD trees than in healthy trees. Further work is required to understand these physiological changes during recovery from TPD.
Beneficial effects in response to application of SWCE exceeded the direct effect of its nutrient content. SWCE had lower N, P, K, micronutrients, and amino acids contents, suggesting that the compost extract could be classed as a biostimulant. Biostimulants enhance endogenous plant processes, beyond the direct effects of their constituents such as nutrients and anti-fungal, anti-microbial, or phytohormonal compounds [29]. There is growing evidence demonstrating the potential of various organic substances, including amino acids mixtures, to increase crop productivity and ameliorate crop tolerance to abiotic stresses [30]. Colla et al. [31] demonstrated the biostimulant actions of a protein hydrolysate containing amino acids and small peptides, which elicited gibberellin- and auxin-like activities, enhancing nitrogen uptake and crop performance of lettuce plants (Lactuca sativa). Perennial Rye-grass (Lolium perenne L.) treated with hydrolyzed amino acids and subjected to high temperatures (36 °C) had improved photosynthetic efficiency [32]. Application of Megafol, a biostimulant containing amino acids and protein to tomato plants under drought stress enhanced induction of a number of drought responsive genes [33]. Our previous trial using watery fish-enriched compost, which may have contained amino acids, also demonstrated, to a lesser extent, the recovery of partial TPD. Amino acids and their metabolites are known to play essential roles during signaling processes as well as in plant stress responses [30, 34, 35]. Exogenous low-dose amino acids such as glutamate, cysteine, phenylalanine, and glycine enhanced the activity of the antioxidant enzymes in soybean [36]. Treatment of rice roots with glutamate induced systemic disease resistance against rice blast by regulating salicylic acid signaling pathway in rice leaves [37].
CONCLUSION
The results from this study suggest that curative treatment is necessary to suppress TPD syndrome development. Bark treatment with SWCE consistently reduced dry cut length and increased latex yield in TPD affected trees. Bark treatment is more effective during the early stages of the syndrome. These findings suggest that SWCE containing amino acids has the potential to be used as an early curative treatment for TPD.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors received funding under the Sriwijaya University Priority Applied Research Project 023/SP2H/LT/DPRM/II/2016 and 102/SP2H/LT/DPRM/IV/2017.


