All published articles of this journal are available on ScienceDirect.
Response of Selected Maize Inbred Germplasm to Maize Lethal Necrosis Disease and Its Causative Viruses (Sugarcane Mosaic Virus and Maize Chlorotic Mottle Virus) in Kenya
Abstract
Background:
Maize lethal necrosis (MLN) disease continues to reduce the productivity of maize drastically threatening food security in the affected regions. It continues to cause yield loss of 30–100 percent in farmers’ fields, depending on the time of infestation which is valued at $198 million in Kenya. This has not only threatened regional trade, but also seed industry. It has been reported in the major maize belts of Uasin Gishu, Trans-Nzoia, Bomet, Narok and Nandi Counties. MLN is caused by the synergistic interaction between Sugarcane Mosaic Virus (SCMV) and Maize Chlorotic Mottle Virus (MCMV). The disease has then spread to other Eastern and Central African countries with devastating food security and economic consequences.
Objectives:
This study highlights result after screening selected maize inbred lines for resistance to MLN, SCMV and MCMV in identifying promising lines for integration into the breeding program for MLN resistance.
Methods:
Sixty-five (65) maize genotypes were artificially inoculated using virus strains collected from Bomet County in Kenya at 3-4 leaf stage. Data on disease severity and incidence, AUDPC and flowering were recorded.
Results:
From the result, the inbred lines had significant differences for SCMV, MCMV and MLN reactions. Based on Area Under Disease Progress Curve (AUDPC) score and ELISA analysis, genotypes MLN001 and MLN006 have the lowest score of 270, whereas OH28 had a maximum at 1259 under MCMV. Genotypes MLN042 and MLN041 were identified as the most promising sources of resistant against SCMV. However, no genotype was identified to have acceptable levels of tolerance to MLN, but MLN001 and MLN013 were identified as the best performers under MLN. This study also validated the presence of MLN tolerance in MLN013 (CKDHL120312) and MLN001 (CKDHL120918) as earlier reported by CIMMYT. These tolerant genotypes are now serving as donors in the introgression of the tolerance into the Kenyan adapted maize backgrounds and development of improved MLN tolerant varieties. This will go a long way in restoring and ensuring sustainable maize productivity in improving the livelihoods of the smallholder farmers who form 75% of the major maize producers in Kenya.
Conclusion:
The identified inbred lines would be recommended for use in varietal development, MLN management and to enhance maize productivity, in the MLN endemic regions and further research in understanding the mode of gene action for MLN tolerance.
1. INTRODUCTION
Maize accounts for > 20% of total agricultural production, and 25% of agricultural employment in Kenya [1]. Thus, Kenya’s national food security is strongly linked to the production of adequate quantities of maize to meet an increasing domestic demand [2, 3]. The total land area under maize in Kenya is about 1.5 million ha, with 70-80% of maize being produced by small-scale farmers with an average on-farm production of 1.5-2.6 tons per ha.
The major biotic causes of stress in maize include Striga a parasitic weed, insect pest, diseases mainly northern corn leaf blight, maize lethal necrosis (MLN), Maize streak virus (MSV), and common leaf rust, gray leaf spot (GLS), stalk and ear rot.
MLN is a new disease in Kenya with its first incidences noticed in 2012 [4-6]. Earlier the disease was observed in Kansas, USA, in 1978 Niblett and Claflin where it was identified as corn lethal necrosis (CLN) disease [7]. Both MLN and CLN are caused by double infection of maize plants by maize chlorotic mottle virus (MCMV) (Machlomovirus: Tombusviridae) in combination with any of the cereal viruses in the Potyviridae group, Sugarcane mosaic virus (SCMV) (Potyvirus: Potyviridae), Maize dwarf mosaic virus (MDMV) or Wheat streak mosaic virus (WSMV). MCMV is transmitted by vectors such as Thrips (Frankliniellawilliamsi Hood) and beetles [8] while SCMV is transmitted by Aphids [9]. Transmission and spread of MCMV have also been reported to be through seeds from infected plants [10] at a rate of 0.0003% which can translate into a high number of infected plants resulting in epidemics. MCMV is a threat on its own and may cause significant yield loss even in the absence of the other viruses.
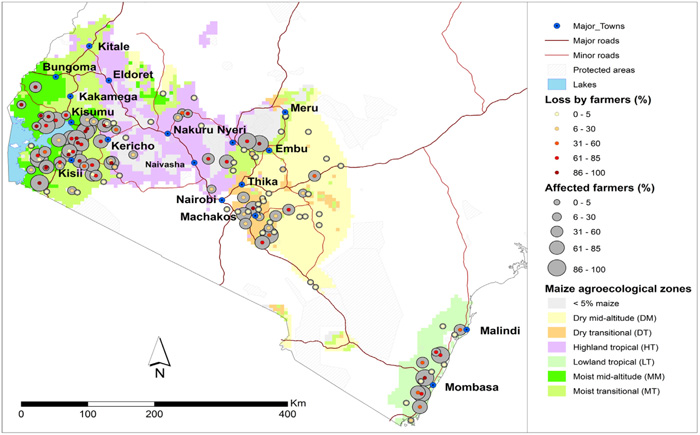
Source: De Groote et al,. 2016
Since its first reports in Bomet county in 2011, the disease has spread into other areas [5] (Fig. 1). Yield losses of up to 100% which is an estimated grain loss of 126, 000 metric tonnes valued at $52 million have been reported in Kenya [2] while Castillo-loayza [11] reported yield losses of up to 59% due to MCMV in Peru. A survey conducted in the maize growing regions of Kenya in 2013/2014 indicated that 60% of the 2,467 randomly selected samples were positive for MCMV with more than 40% of these being infected with MCMV alone. In DRC, MCMV was detected in 40 to 80% of symptomatic samples collected from the Beni, Lubero, and Rutshuru territories of North Kivu Province in 2013 [12, 13]. Other than seed, MCMV has also been reported to be transmitted through soil with 70% of emerging plants found to be infected [12].
Depending on the maize variety, a number of viruses infecting the plant, part of the plant infected, time of infection and prevailing environmental conditions, infected plants show a wide range of symptoms [14]. Common symptoms include; chlorotic mottle on the leaves usually starting from the base of the young leaves in the whorl and extending upwards towards the leaf tips, mild to severe leaf mottling, dwarfing and premature aging of the plants, necrosis of young leaves in the whorl before expansion leading to a “dead heart” and drying up of the whole plant. Severely affected plants form small cobs with little or no grain set. The entire plant can frequently be killed before flowering [5, 15-17] (Fig. 2).
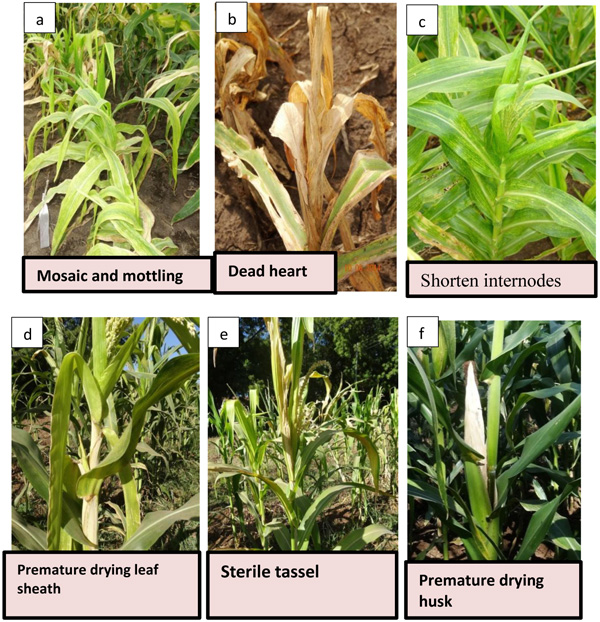
Poor agricultural practices like leaving infected maize crop residues in the field, relay maize planting and maize monoculture aid in inoculum build-up which increases the disease transmission season after season. In addition, poor crop rotation and lack of proper weed management practices has increased maize susceptibility and also aided in offering alternative hosts to the vectors where the weeds are susceptible to the MCMV and SCMV. The management of MLN disease could be achieved by integrating cultural methods, use of a chemical such as seed dressing and vector control with host resistance breeding. However, use of chemicals is uneconomical and environmentally unfriendly, especially among the resource-constrained smallholder maize farmers.
Other measures include; effective monitoring, rigorous implementation of the maize-free period (at least 3 months), timely planting, and use of certified, MCMV-free seed and crop rotation with non-cereal crops [18]. However, these approaches are difficult to implement in Bomet, Kisii and Narok counties where relay planting of maize is a common practice due to frequent rainfalls. Therefore, the most effective control method for MLN, SCMV and MCMV, is the use of resistant maize genotypes.
Development of virus-resistant maize germplasm and hybrids is the most cost-effective and environmentally friendly approach to control disease [19, 20]. The utilization of disease nurseries and trials in the screen houses and field infestation is the easiest approach to screen for MLN resistance in maize.
The objective of this study was to identify sources of resistance to MCMV, SCMV and MLN in a set of selected maize genotypes that can support further research for genetic analysis and development of durable resistant maize lines and hybrids with good agronomic performance under MLN.
2. MATERIALS AND METHODS
2.1. Host plants
Seeds of 65 selected maize genotypes were obtained from the Kenya Agricultural and Livestock Research Organization (KALRO), International Maize and Wheat Improvement Center (CIMMYT) [21] and USDA, ARS Corn, Soybean and Wheat Quality Research Unit (CSWQRU) in Wooster, Ohio. The germplasm was selected based on previous studies and data on resistant to other diseases such as maize streak virus, grey leaf spot, Turcicum leaf blight, common leaf rust among others and/or which from previous screening showed indications of resistance or tolerance to MLN. Four yellow maize lines; P405, OH28, N211 and KS23-6 were used as checks where; PA405 is resistant to SCMV but susceptible to MCMV and MLN, OH28 (CI.112-1 X Oh920) X (I11.A x I11.B) which was released in 1943 [22]. and it is susceptible to Maize dwarf mosaic virus (MDMV), SCMV, Wheat streak mosaic virus (WSMV), Maize chlorotic dwarf virus (MCDV), Maize mosaic virus (MMV), and Maize fine streak virus (MFSV) [23-25, 14]was used as a susceptible check while N211 and KS23-6 were used as tolerant checks for both MCMV and MLN [12]. In the second and third screening, germplasm that had a severity score higher than OH28 in MCMV and MLN were removed, leaving only thirty genotypes. Plastic pots measuring 30 cm in diameter filled with autoclaved (heat-sterilized) silt- loam soil and manure mix (6:1, v/v) were arranged in a randomized complete block design of three replications. Seeds were sown at a rate of five seeds per pot, giving a total of 15 plants per genotype. Di-Ammonium Phosphate (DAP) and Calcium Ammonium Nitrate (CAN) were applied to give 60kgN and 60kgP2O5 ha-1 at planting and at 8 weeks after planting (WAP) respectively.
2.2. Virus isolates/ Inoculum preparation and inoculation
MCMV and SCMV isolates used in this study were originally collected from Bomet County in South Rift valley in Kenya and have been maintained at KALRO-Kabete, National Agricultural Research Laboratories (NARL) by serial passage on to susceptible maize hybrid H614 in separate greenhouses. Virus strain identity was verified at each passage time inoculum was prepared for a test by also inoculating susceptible maize germplasm OH28 and H614, Sorghum bicolor (L.) Moench cv. Atlas and Sart. Atlas and Sart sorghums are resistant to MCMV while Sart is susceptible to MDMV and SCMV but resistant to WSMV [16]. As in other crops, it is very difficult to diagnose virus diseases in maize-based solely on symptoms, as these vary significantly based on plant genotype, time of infection, environmental conditions and the potential for multiple infections. Therefore, the serological assay, ELISA, was used to check the Virus purity, inoculation and disease assessment for MCMV and SCMV.
At 3 to 4 leaf stage (10 days after planting) all plants were mechanically inoculated twice within a 1-week interval by rubbing the 2 youngest leaves [26]. Virus inoculum was prepared from freshly harvested infected symptomatic young plants (infected 10-15 days prior to the main inoculation). Before inoculation, leaves were homogenated in 0.01 M phosphate buffer (pH 7.0) in 1:8 dilutions and 0.6% of 22µm carborundum were added prior to inoculation. The inoculum was kept in ice during inoculation time. The plants were watered daily until all the plants flowered.
2.3. Symptoms identification/rating
Disease incidences, severity and 50% days to anthesis (pollen shade) were recorded. Plants were diagnosed for virus symptoms from 7 days post-inoculation (dpi) every 2 days interval up to 56 days, using a 1 to 5 scale, where; 1= no symptoms, 3= mild symptoms and 5= Severe chlorosis (dieback of the plant) (Fig. 3). Included in the diagnoses were notations of whether symptoms were local lesions on inoculated leaves or systemic infections that were limited or general and consisted of mosaics, mottles, or flecks and streaks.

Double-antibody sandwich ELISA (DAS-ELISA) analysis was carried out to confirm virus identity prior to inoculation and non-symptomatic plants. 500 mg of tissue were taken from the tip of 6th and 7th leaf and homogenized in 2.5 ml of 1x extraction buffer; PBST, pH 7.2 (137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4), containing 2% BSA and 0.05% Tween 20. The ground samples were placed at –20°C for long-term storage. The samples were thawed to room temperature before loading plates. Plates were coated with a 1:1000 dilution of virus-specific IgG (1mg/ml) in carbonate coating buffer, pH 9.6, and the secondary antibody; alkaline phosphatase conjugated IgG (1 mg/ml, Sigma) at a 1:5000 dilution, both of which proved to be optimal in a previous evaluation. After incubation of the plates containing a primary antibody for 1 hour at 37°C, the wells were washed with 200 µl of PBST, 200 µl of the diluted samples were added and plates were incubated at 37°C for 1 hour. Washing was done as above and secondary antibody was added, plates were incubated for 1 hour at 37°C. Finally, 200 µl of a 0.6 mg/ml solution of p-nitrophenyl phosphate (Sigma) was added to each well and color development was allowed to continue for 1 hour at room temperature. ELISA Nunc (Inter Med., Denmark) microtiter plates were used and absorbance was recorded at 405 nm in an MR700 Dynatech spectrophotometer (Dynatech, UK). Blank, buffer, positive control from the primary inoculum and health sample were used on all plates as controls. The readings were carried out at 60 minutes after addition of the substrate. In cases of doubt, the reading overnight was compared to the reading at one hour. A sample was judged as positive when the reading was greater than three times the absorbance value of a healthy (negative) plant extract reaction.
3. STATISTICAL ANALYSIS
Analysis of variance for each trial and combined analysis across years was performed using Multi-environment Trial analysis with R; version 5.0, GenStat 15th edition and Breeding management system software, version 3.0.9 to determine the level of significant difference between genotypes. Area Under the Disease Progress Curve (AUDPC) which is a better indicator of disease expression over time was calculated on a single plant basis by trapezoidal integration over the whole observation period as follows:
AUDPC= ∑i[ (DSi + DSi-1) x (ti-ti-1)]/2
Where “i” ={6, 8, 10, 12, 14, 16, 18, 20, 22, 31, 38, 45, 56} are the days of observation, “DS” is the disease score using the above severity score of 1 to 5 and “t” represents the number of days post-inoculation [27, 28]. Genotypic correlations (Rg) between treatments were estimated according to Coopre et al., 1996 as:
Rg = Rp(12)/(H1 x H2)1/2
In which Rp(12) is the phenotypic correlation between the traits measured in treatments 1 and 2, H1 and H2 are the broad-sense heritability for the trait measured in screen houses 1 and 2, respectively.
4. RESULTS
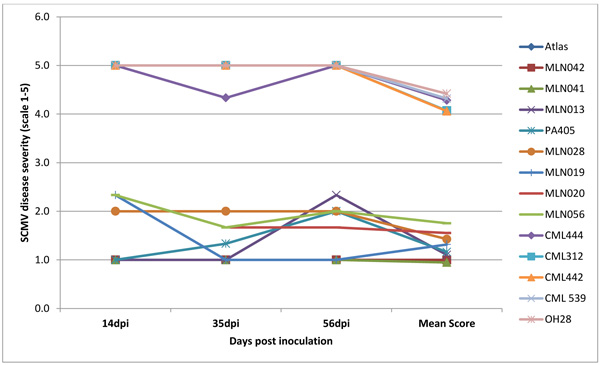
Infection was observed in all the inoculated plants but the maize lines differed significantly (P <0.001) for the resistance to SCMV, MCMV and MLN. Disease severity differed significantly with plant growth with first disease symptoms in susceptible genotypes observed at 5 to 6 days post-inoculation (dpi) for SCMV, 10 to12 days for MLN and 14 to 15 days for MCMV. Disease severity was rated up to 56 dpi a time at which most of the lines had attained 50% flowering. There was significant genetic variation (p≤0.001) among the genotypes at 14, 35 and 56 dpi for disease severity, mean disease score and area under disease progress curve (AUDPC) (Figs. 4, 5, 6 and Table 1). Only 17% of the total germplasm screened under SCMV had a mean score < 2.5. Two maize genotypes; MLN041 and MLN 042 had the lowest AUDPC value of 286 compared to mean AUDPC value of 1338.4 for the susceptible germplasm Table 1.
| MCMV | SCMV | MLN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pedigree | AUDPC | Days to 50% Anthesis | Days to 50% Silking | Pedigree | AUDPC | Days to 50% Anthesis | Days to 50% Silking | Pedigree | AUDPC |
| MLN001 | 270 a | 71.33 abcdefgh | 73.67 abcdefghijk | Atlas | 286 a | 68 bcdefghijkl | 73.67 defghijklm | Atlas | 196 a |
| MLN006 | 270 a | 59.67 abc | 62 abc | MLN042 | 286 a | 66 abcdefghij | 69 abcdefghij | MLN001 | 471.3 ab |
| Atlas | 270 a | 70 abcdefgh | 74.33 abcdefghijk | MLN041 | 288 a | 69 bcdefghijklm | 70.67 bcdefghijklm | MLN013 | 518 abc |
| MLN012 | 286 a | 75 bcdefgh | 77 abcdefghijk | MLN013 | 321 ab | 77.33 klm | 80.67 klmn | MLN006 | 535.5 abcd |
| MLN016 | 287 a | 81 abcdefgh | 83 abcdefghijk | MLN019 | 378 abc | 71.33 cdefghijklm | 70.33 bcdefghijkl | MLN019 | 551.2 bcde |
| MLN008 | 292 a | 77.33 bcdefgh | 78.67 bcdefghijk | PA405 | 385 abc | 61.67 abcd | 62.67 abcd | MLN052 | 555.9 bcdef |
| MLN007 | 298 a | 76 bcdefgh | 82.67 cdefghijk | MLN028 | 453 abcd | 70.67 cdefghijklm | 73 defghijklm | MLN016 | 565.8 bcdef |
| N211 | 454 ab | 64.67 abcdefg | 66.33 abcdef | MLN020 | 474 abcde | 63 abcdefg | 60 ab | MLN018 | 573.4 bcdefg |
| MLN009 | 459 ab | 72.33 abcdefgh | 75 abcdefghijk | MLN056 | 566 abcdef | 66 abcdefghij | 70.33 bcdefghijkl | MLN002 | 582.8 bcdefgh |
| MLN050 | 636 bc | 58.33 ab | 59.33 ab | CML444 | 1311 hi | 73.67 ghijklm | 74.33 efghijklmn | MLN056 | 601.1 bcdefgh |
| KS23-6 | 844 cd | 77.33 bcdefgh | 77.67 abcdefghijk | CML312 | 1323 i | 70 cdefghijklm | 70.33 bcdefghijkl | KS23-6 | 606.7 bcdefgh |
| CML312 | 1132 fgh | 70.67 abcdefgh | 73.33 abcdefghijk | CML442 | 1326 i | 70.67 cdefghijklm | 73.33 defghijklm | N211 | 663.8 bcdefgh |
| CML444 | 1166 fgh | 84.33 fgh | 86 efghijk | CML 539 | 1363 i | 66 abcdefghij | 68.67 abcdefghij | CML444 | 666.8 bcdefgh |
| CML 539 | 1165 fgh | 69.33 abcdefgh | 71.33 abcdefghij | OH28 | 1369 i | 69.67 cdefghijklm | 72.33 defghijklm | CML312 | 722.2 bcdefgh |
| CML442 | 1178 fgh | 76.33 bcdefgh | 75.67 abcdefghijk | MLN006 | 960bcdefghijk | 58.67ab | 63.76abcde | CML 539 | 890.2 efgh |
| PA405 | 1182 fgh | 67.33 abcdefgh | 70.67 abcdefghij | OH28 | 913.5 gh | ||||
| OH28 | 1259 h | 73.33 abcdefgh | 75 abcdefghijk | CML442 | 922.8 h | ||||
| Mean | 1019.94 | 73.099 | 76.12 | Mean | 1116.7 | 69.932 | 72.609 | Mean | 740.38 |
| s.e. | 69.15 | 5.993 | 6.159 | s.e. | 196.7 | 3.142 | 3.352 | s.e. | 79.94 |
| cv% | 6.8 | 8.2 | 8.1 | cv% | 17.6 | 4.5 | 4.6 | cv% | 10.8 |
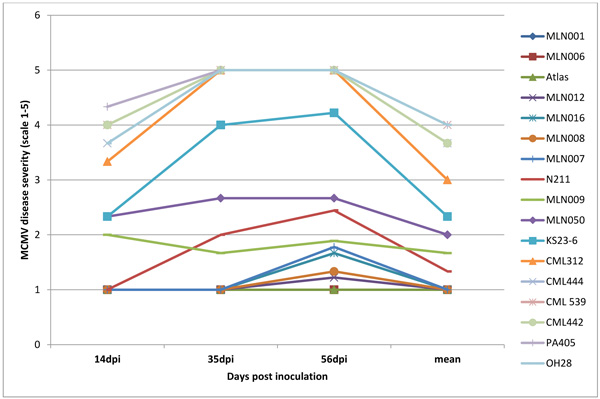
The symptoms were first observed at 14 dpi from the lower leaves and spread slowly to the newly emerging leaves in the MCMV experiment. At 20dpi, 45% of the germplasm screened had a disease severity score < 3, while at 56dpi, only 15% of the germplasm were <3. Only 9% of the total germplasm in the 2 years period of screening showed acceptable levels of tolerance to MCMV. Among the top-performing were; MLN001, MLN006 MLN012, MLN016, MLN008, MLN007 and MLN009 which had a disease severity score <2.0 at 56dpi. N211, unlike the others, developed symptoms slowly with the first symptoms been observed at 20 dpi and with moderate symptoms at 56dpi (Fig. 5).
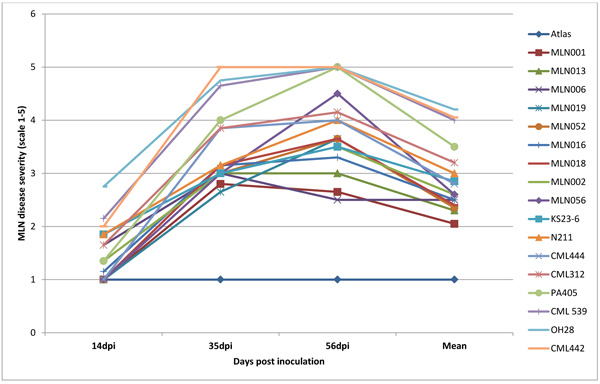
Nearly all plants inoculated with MLN (SCMV and MCMV combined) developed symptoms within 10 dpi. At 35dpi, 95% germplasm screed had a mean disease severity score of < 2.5. However, symptoms for genotypes MLN001 and MLN006 developed moderately with a final score of < 3.0 at 56 dpi (Fig. 6). The disease severity on susceptible germplasm was high and reached a maximum score of 5 and highest AUDPC value of >650 Table 1. More than 60% of germplasm under MLN showed stunted growth and dead heart (Fig. 2b) or died before reaching knee height. Since the plants were allowed to grow to maturity, >50% of the germplasm did not tussle. For those that managed to produce tassel, they did not shed pollen (sterile) (Fig. 2e). Majority of the germplasm did not produce silk. The tolerant germplasm N211, KS23-6, MLN001, MLN006, MLN013 and MLN019 managed to tassel and produced silk. However, the ears were either deformed, partially filled or had husk cover senesced prematurely. If a maize field is infected with MLN late in the growing cycle, an increased dry husk (Fig. 2f) and a high number of rotten ears are observed (Karanja et al unpublished).
Other than Sart and Atlas sorghums, no other germplasm was found to be completely immune to MCMV based on ELISA tests Table 2. However, N211 and MLN016 can be considered to be moderately tolerant to MCVM given the low ELISA readings Table 2. Moreover, inbred MLN013, MLN041 and MLN019 were found to be resistant to SCMV as no virus was detected with ELISA analysis. In germplasm MLN042, MLN020, PA405 and CML 444, SCMV virus were detected when analysed with ELISA suggesting low virus titter hence tolerance to SCMV.
| MCMV | SCMV | ||||
|---|---|---|---|---|---|
| Genotype | Elisa Reading | Reaction Type a | Genotype | Elisa Reading | Reaction Type a |
| Buffer | 0.18 | MLN013 | 0.09 | R | |
| Atlas | 0.25 | R | MLN041 | 0.09 | R |
| Health control (*3) | 0.41 | MLN019 | 0.09 | R | |
| MLN006 | 0.42 | T | Buffer | 0.09 | |
| N211 | 0.47 | T | MLN042 | 0.1 | R |
| MLN016 | 0.66 | S | Atlas | 0.1 | R |
| CML442 | 0.74 | S | MLN020 | 0.11 | R |
| MLN009 | 0.74 | S | PA405 | 0.13 | R |
| OH28 | 0.87 | S | CML444 | 0.15 | R |
| MLN007 | 0.94 | S | OH28 | 0.2 | T |
| MLN001 | 0.95 | S | CML442 | 0.23 | T |
| CML 539 | 1.03 | S | MLN028 | 0.24 | T |
| MLN012 | 1.03 | S | CML 539 | 0.25 | T |
| CML312 | 1.09 | S | CML312 | 0.25 | T |
| CML444 | 1.11 | S | Health Control (*3) | 0.26 | |
| Positive control | 1.15 | Sart | 0.28 | Highly Susceptible | |
| CML395 | 1.2 | Highly Susceptible | MLN006 | 0.29 | Highly susceptible |
| MLN050 | 1.25 | Highly Susceptible | Positive control | 1.07 | |
| Heritability | 0.85 | 0.96 | |||
| Mean | 0.86 | 0.2 | |||
| LSD0.05 | 0.38 | 0.08 | |||
| CV (%) | 25.17 | 22.66 | |||
However, there is a need to use real-time quantitative PCR (RTqPCR) to confirm these given the low sensitivity of ELISA.
A negative genetic and phenotypic correlation was observed under MLN between 56 days post inoculation (dpi), disease severity score pooled mean (dspm), Area Under Disease Progress Curve (AUDPC) and 50% days to anthesis (Anth) and silking (SLK) Table 3. Most of the plants in MLN experiment were stunted or died resulting in missing data on anthesis and silking, thus high cv(%) of 51.2 Table 1. However, the majority of plants under SCMV and MCMV managed to flower despite the severe symptoms even for the checks Table 1.
| a) MCMMV | |||||
|---|---|---|---|---|---|
| Traits | 52dpi | dspm | AUDPC | Anth. | Slk |
| 52dpi | 1 | 0.92*** | 0.99*** | 0.11* | 0.12* |
| dspm | 0.95*** | 1 | 0.96*** | 0.16* | 0.16* |
| AUDPC | 0.99*** | 0.99*** | 1 | 0.13* | 0.14* |
| Anth. | 0.14* | 0.20* | 0.17* | 1 | 0.93** |
| SLke | 0.15* | 0.20* | 0.17* | 1.00 | 1 |
| b) MLN | |||||
| 52dpi | 1 | 0.89*** | 0.93*** | -0.51** | -0.60** |
| dspm | 0.92*** | 1 | 0.99*** | -0.48* | -0.52** |
| AUDPC | 0.96*** | 0.99*** | 1 | -0.51** | -0.56** |
| Anth | -0.57** | -0.52** | -0.56** | 1 | 0.80*** |
| Slk | -0.73** | -0.62** | -0.67** | 0.93*** | 1 |
| c) SCMV | |||||
| 52dpi | 1 | 0.97*** | 0.99*** | 0.04ns | 0.08ns |
| Mean | 0.97*** | 1 | 0.99*** | 0.03ns | 0.06ns |
| AUDPC | 0.99*** | 0.99*** | 1 | 0.04ns | 0.07ns |
| Anth | 0.04ns | 0.03ns | 0.04ns | 1 | 0.96** |
| Slk | 0.09ns | 0.07ns | 0.08ns | 0.98*** | 1 |
5. DISCUSSION
The analysis of disease severity revealed that the genotypic effect of SCMV, MCMV and MLN was highly significant on all assessment/scoring dates as well as on the pooled mean and AUDPC values. The susceptible genotypes; CML444, CML 312, CML442, CML 539 and OH 28, showed the highest disease scores at all assessment dates, pooled mean and AUDPC. Other than Atlas sorghum, no maize genotype was observed to be immune to MLN. Evaluation of the germplasm suggests that MLN041 and MLN042 may be carrying the desirable genes for SCMV resistance and MLN001, MLN006, MLN016 and N211 for MCMV tolerance. This study has also confirmed that PA405 developed in Pennsylvania is tolerant to SCMV strain used in this study. Inbred PA405 was reported to show complete resistance to MDMV and SCMV inoculation under both field and greenhouse conditions [29] with six major genes conferring resistance to SCMV and MDMV being identified [30-34]. Further studies need to be conducted in MLN042 and MLN041 to confirm and identify the number of genes conforming to resistance Inbred N211 which showed delayed symptoms expression with the first symptoms appearing at 20 dpi could be considered have partial tolerant to MCMV since a low viral load was detected at 25 dpi using ELISA. The delayed symptoms development in N211 was also observed by [12]. Similar observations were also made by Kovacs [35] after screening different maize inbred lines and hybrids with MCMV. Nelson [36] also observed that the level of resistance to MCMV varied widely among the maize lines screened suggesting that MCMV resistance is controlled by many minor genes. The extended incubation period observed in N211 is an indication of the presence of resistance genes which are providing a certain degree of MCMV resistance. Similar results of delayed symptoms were observed under MLN for the inbred N211, KS23-6, MLN016 and MLN009. This could be associated to reduce synergistic interaction between SCMV and MCMV gave their high level of tolerance to MCMV [37]. Zihao [38] reported that the co-infection of MCMV and SCMV increases the accumulation of MCMV [38]. The accumulation of MCMV genomic RNAs and the expression of MCMV CP have been observed to be higher in MLN-infected leaves than single-infected leaves, with no obvious difference observed for SCMV RNA and SCMV CP expression levels between MLN and single-infected leaves [38]. This suggests that the symptoms expression in MLN for the inbred MLN042 and MLN041 were for MCMV given their high level of resistance to SCMV. This study also validated the presence of MLN tolerance to MLN013 (CKDHL120312) and MLN019 (CKDHL140918) as earlier reported by CIMMYT [39]. However, more study needs to be conducted on these germplasm together with N211, KS23-6, MLN001, MLN006, MLN009 and MLN016 to understand the mechanism underlying the synergistic interaction between MCMV and SCMV. The failure of >50% of screened germplasm to reach flowering and/or delayed flowering, male sterility, poorly filled cobs and a high number of rotten ears under MLN trials is an indication of the high impact of the disease on yield.
CONCLUSION
The results presented here reveal that the studied maize germplasm has sufficient variability for response to SCMV, MCMV and MLN. SCMV seems to play an important role in increasing MLN symptoms in MCMV susceptible maize genotypes and can be considered as a catalyst to its multiplication. This indicates that in developing MLN tolerant/resistant maize varieties, more focus should be on identifying and using SCMV tolerant maize germplasm. These tolerant genotypes are now serving as donors in the introgression of the tolerance into the Kenyan adapted maize backgrounds and development of improved MLN tolerant varieties combined with multiple abiotic and biotic stress tolerance traits. This will go a long way in restoring and ensuring sustainable maize productivity and improving the livelihoods of the smallholder farmers who form 75% of the major maize producers in Kenya.
In addition, the inbred lines should be used to conduct further research on the mechanism responsible for the healthy pollen-diseased silk relationship, SCMV and MCMV synergism as earlier suggested by Mikel [40].
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank KALRO, CIMMYT and USDA-ARS for providing seeds of inbred lines used in this study. We are grateful to KAPAP and CIMMYT for proving the funds to support this study.


