All published articles of this journal are available on ScienceDirect.
Enhancing Non-symbiotic N2 Fixation in Agriculture
Abstract
Much of the demand for nitrogen (N) in cereal cropping systems is met by using N fertilisers, but the cost of production is increasing and there are also environmental concerns. This has led to a growing interest in exploring other sources of N such as biological N2 fixation. Non-symbiotic N2 fixation (by free-living bacteria in soils or associated with the rhizosphere) has the potential to meet some of this need especially in the lower input cropping systems worldwide. There has been considerable research on non-symbiotic N2 fixation, but still there is much argument about the amount of N that can potentially be fixed by this process largely due to shortcomings of indirect measurements, however isotope-based direct methods indicate agronomically significant amounts of N2 fixation both in annual crop and perennial grass systems. New molecular technologies offer opportunities to increase our understanding of N2-fixing microbial communities (many of them non-culturable) and the molecular mechanisms of non-symbiotic N2 fixation. This knowledge should assist the development of new plant-diazotrophic combinations for specific environments and more sustainable exploitation of N2-fixing bacteria as inoculants for agriculture. Whilst the ultimate goal might be to introduce nitrogenase genes into significant non-leguminous crop plants, it may be more realistic in the shorter-term to better synchronise plant-microbe interactions to enhance N2 fixation when the N needs of the plant are greatest. The review explores possibilities to maximise potential N inputs from non-symbiotic N2 fixation through improved management practices, identification of better performing microbial strains and their successful inoculation in the field, and plant based solutions.
1. INTRODUCTION
Non-symbiotic (NS) N2 fixation includes N2 fixation by free-living soil bacteria (autotrophic and heterotrophic) that are not in a direct symbiosis with plants, and associative N2-fixation (e.g. associated with the rhizospheres of grasses and cereals). Free-living N2 fixation can also be associated with decomposing plant residues, aggregates with decomposable particulate organic matter and in termite habitats. A conceptual diagram of the different NS N2-fixing possibilities and their relationship with soil N cycle is presented in Fig. (1).
Globally, the demand for N fertilisers is expected to exceed 112 million tonnes in 2015 [1] and much of this is produced by the Haber-Bosch process [2], a process which uses large amounts of fossil fuel [3]. This, together with the increasing demand for organically grown agricultural and horticultural products, and the need to address economic and environmental concerns, has rekindled interest in promoting biological N2 fixation in non-leguminous crops. Nitrogen is a critical element for sustainable agriculture but inappropriate use of fertiliser results in lower efficiency and has the potential to contribute to (1) greenhouse gas loads such as N2O thereby contributing to climate change, and (2) leaching of N from agricultural lands as NO-3 causing eutrophication of rivers, lakes and oceans, and reducing the quality of water supplies [4]. Since 1993, the use of N fertilisers in Australia has more than doubled (1.314 million tonnes of N in 2013 [5]) and more than doubled in China in the last 25 years [4]. The need to maximize the nutrient (N) inputs from natural processes such as biological N2 fixation is greater today than ever before [6]. Recent increases in fossil fuel costs have resulted in significant increases in N fertiliser costs and the increased variability in rainfall patterns escalates the risk associated with higher input costs in the rainfed farming systems. Cleveland et al. [7] estimated that the potential global biological N2 fixation (symbiotic and NS) in natural ecosystems is between 100 and 290 million tonnes N year-1. In soils under agricultural production, estimates of biologically fixed N range from approximately 33 million tonnes N year-1 [8] to 50-70 million tonnes N year-1 [9].
This review summarizes the current knowledge on NS N2 fixation, including measurement techniques, factors that control the function, ecology of N2-fixing bacteria and identifies opportunities to harness this biological process for production and environmental benefits.
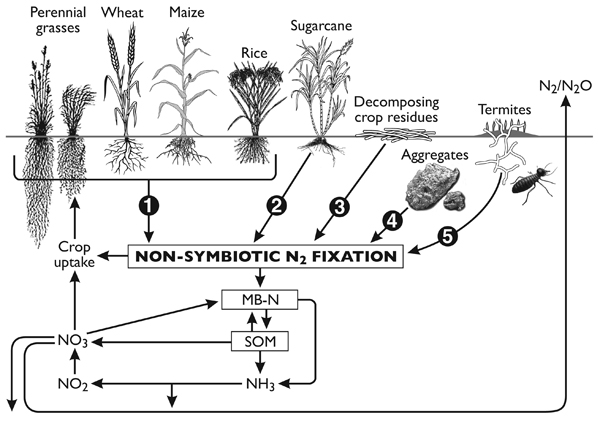
A conceptual diagram highlighting the diverse set of microsites that can support N2 fixation by NS N2-fixing bacteria and its role in the soil N cycle. (1) A diverse array of bacterial genera have been found in the rhizosphere and endophytic environment of a variety of cereals and other crop plants; the amount of N2 fixation in the field environment is yet to be properly quantified, (2) A significant amount of N2 fixation has been shown to occur in the below- and above-ground plant environments with sugarcane, (3) fresh decomposing residues, especially with wide C:N ratios, provide optimal conditions for N2 fixation, (4) stable aggregates not only provide protective sites for free-living, N2-fixing bacteria but also provide the required low oxygen conditions for nitrogenase activity, and (5) a diverse group of microflora in termite guts and nests possess nifH genes that show potential for N2 fixation in natural environments mainly in semi-arid and arid ecosystems.
2. CROP N DEMAND AND SUPPLY
It takes ~26 kg N to produce 1 t of wheat grain including straw [10]. Of this ~20 kg N is removed in every tonne of grain harvested, and hence, for grain yields between 2-8 t ha-1, this represents an annual removal of 40-160 kg N ha-1 [11]. At peak demand in a growing crop, N demand exceeds supply from N mineralisation [10] and N fertilisers compensate for much of this shortfall.
In Australia since 2001, wheat yields averaged ~1.7 t/ha [5] and, based on the calculations above, this represents an average annual N removal of ~ 32 kg N ha-1. N fertiliser use across Australia ranged from 0.7 million tonnes in 2003 to 1.34 million tonnes in 2013 [5]. If biological systems could be manipulated to increase the inputs of N from NS N2 fixation it should be possible to reduce the requirement for industrially fixed N fertilisers.
Agronomically-significant amounts of N2 fixation (25-50 kg N ha-1 year-1) have been measured in C4 grasses including sugar cane [12-16], but quantification of N2 fixation associated with cereals under natural conditions is limited [12]. Kennedy and Islam [17] concluded that 10-30 kg N ha-1 crop-1 could be fixed by free-living and rhizosphere N2-fixing bacteria associated with wheat, and similar ranges were indicated by Dart [18] for cereal production systems in temperate and tropical environments. Field measurement of N2 fixation by free-living bacteria using cereal residues as an energy source indicated 1-12 kg N ha-1 fixed during short periods of 2-4 weeks [19-21], but where warm, moist conditions coincide with fresh stubbles, annual potentials of up to 38 kg N ha-1 year-1 have been calculated [22]. Giller and Merckx [23], on the other hand, were less optimistic and estimated that inputs from NS N2 fixation are likely to be <5kg N ha-1 year-1. Kennedy and Islam [17] calculated that, based on an average yield of straw of 2 t ha-1 with a C content of 43%, a contribution of 50-150 kg N2 ha-1 fixed by free-living bacteria is theoretically possible, provided that metabolism of the straw is substantially directed towards N2 fixation.
Most estimates of NS N2 fixation have been determined by indirect measures such as C2H2 reduction or calculation from N balance. Rates of NS N2 fixation are significantly less than estimated N inputs from symbiotic N2 fixation which range from 2 to 284 kg N ha-1 year-1 in legume pastures [24] and 0-271 kg N ha-1year-1 in grain legumes [25, 26].
Many of the measurements of NS N2 fixation were made more than 20 years ago. Since then, across the world, there have been significant changes towards the adoption of conservation farming practices. No-tillage and stubble retention practices have been widely adopted [27, 28]. Farming systems worldwide during this time have moved towards intensive cropping systems and in particular intensive cereals in Australia. These changes have resulted in increases in productivity of more than 50% [5] with associated increases in nutrient and carbon turnover. All of these changes are likely to have significant impacts on NS N2 fixation through the provision of larger C resources for biological activity, and the creation and preservation of ideal soil conditions, e.g. habitable microsites for NS N2 fixation.
3. MOLECULAR ECOLOGY OF N2-FIXING (DIAZOTROPHIC) POPULATIONS
More than 50 different genera of culturable diazotrophic bacteria have been identified [17, 29, 30], but more recently, new technologies have expanded our knowledge of NS microbial communities and their function. Molecular technologies utilising analysis of the nifH gene (a structural gene encoding for the highly conserved nitrogenase reductase) and stable isotope (15N2) probing have identified a suite of previously unrecognised diazotrophic microorganisms and helped to unravel the complexity of N2-fixing communities in a range of natural and agricultural ecosystems (e.g. [31-35]). Varying growth requirements of a phylogenetically heterogeneous group of N2-fixing microorganisms have precluded cultivation of a significant proportion of these organisms. Notwithstanding this, there is a significant body of work cited by Buckley et al. [32] which suggests that these non-culturable diazotrophs may be the dominant components of N2-fixing communities in soils compared with their culturable cousins.
The great diversity of diazotrophic microorganisms ensures the adaptability of populations of N2-fixing microorganisms to a wide range of conditions. This is reflected in the studies by Bürgmann et al. [36] and Zhang et al. [37] who observed that at any one time, those organisms actively fixing N2 represented only a very small subset of the total diazotrophic community. Recent research combining the use of isotopes with molecular studies is shedding new light on changes in the structure of free-living N2-fixing communities in soils and relating this to function [38]. This type of diversity analysis should help identify which members of the bacterial community are contributing to the soil N cycle in different crops and environments.
The nitrogenase enzyme is mainly found in Bacteria and Archaea and predominantly in chemotrophs, phototrophs and heterotrophs [33] in soils, termite guts, lakes, rivers, estuaries, algal mats and sediments and oligotrophic oceans. This enzyme complex is encoded by nifH, nifD and nifK genes, although nifH gene has been used as the signature gene for molecular diversity studies. By using the nifH sequences available in public databases, five major clusters with homology to nifH have been described [31, 39, 40]. Gaby and Buckely [40] found that the diversity of diazotrophs, based on nifH sequence homology, is not distributed evenly across phylogenetic groups or environments, and that the majority of this diversity is still undiscovered, particularly in soils and in anaerobic environments. Diversity estimates (Chao 1 richness estimates) indicated that soils account for the highest diversity of diazotroph sequences compared to marine environments. Most diazotroph sequences from the α, β and γ Proteobacteria have been recovered from soils [40]. Members of the Subcluster IA (e.g. members of (Δ-Proteobacteria) account for a large portion of nifH sequences in some soils and are actively involved in N2 fixation [38]. Gupta et al. [41] found that members belonging to α-proteobacteria or Cluster Ik/j were the most abundant group in Australian wheat fields. They suggested that on a continental scale, habitat and environment determine the composition of the diazotrophic community, while plant type and management associated factors drive the composition, genetic potential and NS N2 fixation regionally and within fields.
The use of nifH gene analysis, using nifH amplicon sequencing and nifH microarray methods, of isolated organisms and entire N2-fixing microbial communities at the plant interface (rhizosphere, rhizoplane, and phyllosphere) have all shown that the nifH gene is widely distributed in phylogenetically diverse groups of bacteria and archaea [31, 33, 37, 40] and that a large contingent of these organisms is non-culturable, e.g. in the rhizosphere of graminaceous plants [32, 34, 42].
Other associations of N2-fixing bacteria with plants can be endophytic - both obligate and facultative (e.g. [35, 42-45]), but whether their relationship with the plant is symbiotic or NS is uncertain [45]. Endophytic bacteria are at an advantage compared with free-living or rhizosphere bacteria because they have ready access to carbon (energy source) nutrients and water from within the plant [46] and are not vulnerable to competition from other microorganisms in the rhizosphere or soil. Therefore, such organisms are more likely to be successful as inoculants. As with non-endophyte diazotrophs, non-culturable N2-fixing microorganisms appear to be dominant. From phylogenetic analyses of nitrogenase sequences, Hurek et al. [42] predicted that non-culturable grass endophytes (such as Azoarcus sp.) are ecologically dominant and could play an important role in N2 fixation in natural grass ecosystems.
Termites and termite habitats are important components of soil food webs contributing to soil N cycle in rangelands and low rainfall regions in Australia, Africa and India. Molecular analysis using nifH PCR primers has extended our understanding of the diversity of N2-fixing communities in guts of termites that feed on cellulose substrates [31, 47, 48].
4. MEASUREMENT/QUANTIFICATION
Current methods of quantifying NS N2 fixation are far from perfect and measurement may be flawed if inappropriate techniques or inadequate controls are used. However, used appropriately, these techniques can provide some valuable insight into the role and importance of NS N2 fixation. New molecular technologies promise further advances and this will be addressed later in this review. Advantages and disadvantages of current techniques and their appropriate use are described here briefly.
4.1. C2H2 Reduction Assay
The C2H2 reduction assay (based on the reduction C2H2 to C2H4 by nitrogenase) is a rapid, sensitive, simple and low cost method which if used with appropriate controls and calibrations can be useful for evaluating nitrogenase activity in time and space [49]. Under controlled conditions it can be extremely useful for comparative purposes where absolute values of N2 fixation are not critical. Hardy et al. [50] found a direct correlation between N2 fixation (N2→2NH3) and C2H2→C2H4 in pure cultures of diazotrophs and in legumes, and calculated that the theoretical relationship of C2H2 reduced to N2 fixed was 3. However, measured values of conversion factors in different environments can vary widely; these are summarized in Table 1.
Such variations in ratios of C2H4 produced to N2 fixed have been the basis of most criticism of the acetylene reduction assay, although research has shown that for all systems including pure cultures, legumes, non-legumes and soils that this ratio averaged between 2.6 and 6.9 [51]. The only exception to this was anaerobic soil which had conversion factors of up to 25. Under other conditions a reasonable estimate is possible, although any experimental procedure should always include a calibration of the assay using 15N2 gas exposure [52-54].
4.2. Use of 15N2 Gas as a Direct Measure of N2 Fixation
This method can be sensitive, accurate and provide absolute proof of N2 fixation and has been used to demonstrate N2 fixation associated with cereals and grasses [41, 55, 56] and in soils [19, 56, 57]. It is a most useful method for calibrating other measures of N2 fixation. Although difficulties in controlling environmental conditions can be encountered when using the method to measure N2 fixation associated with plants, it has been successfully used to estimate N2 fixation with cereals and sorghum [55, 56].
Different values used for conversion factor from acetylene reduced to N2 fixed
| Source | Conversion factor | Reference |
|---|---|---|
| Theoretical average | 3 or 4 | Hardy et al. [51] |
| Jenson and Cox [192] | ||
| Legumes, non-legumes and soils | 2.6 - 6.9 | Hardy et al. [51] |
| Anaerobic soil | 25 | Hardy et al. [51] |
| Suspensions of bacteria | 2-8 - 4.7 | Hardy et al. [51] |
| Montoya et al. [193] | ||
| Overall range | 0.56 - 22 | Boddey et al. [59, 61] |
| Anabaena (17.3 oC) | 3.96 | Liengen [111] |
| Anabaena (14.0 oC) | 4.88 | Liengen [111] |
| Cyanobacterial crusts | 0.0216-0.073 | |
| Nostoc community | 0.11-0.48 | |
| Sub-alpine meadow & coniferous forest | 7.5 | Skujins et al. [194] |
| Saline soils | 2.1 - 2.8 | Zechmeister-Boltenstern and Kinzel [195] |
| Peat soils | 5.4 | Zechmeister-Boltenstern and Kinzel [195] |
| Grassland, wheat fields, fallow | 3.1-8.6 | Steyn and Delwiche [52] |
| Forest soils | Nohrstedt [196] | |
| Low water (75% of water saturation) | 2.6 | |
| High water (100% water saturation) | 15.7 | |
| Forest soils | 1.6-5.6 | Nohrstedt [197] |
| Decomposing litter | 3.9 | Vitousek and Hobbie [198] |
| Coastal sediments | 0.11 - 94 | Seitzinger and Gaber [199] |
Demonstrating the incorporation of 15N2 into free-living microbial populations in the soil is much more difficult. While N2 fixation in soils was confirmed both in the laboratory and in the field by incorporation of 15N2 [19, 41], absolute measures of N2 fixation in the field using 15N2 gas are costly and are influenced by seasonal factors (M. M. Roper, G. L. Turner, F. J. Bergersen, unpublished data; [58]). Many free-living, diazotrophic bacteria require reduced oxygen concentrations to fix N2 and are located within microsites of low oxygen tension. Sites that restrict oxygen availability may also limit access by 15N2 and this could result in an underestimation of N2 fixation.
4.3. 15N Isotope Dilution and Natural Abundance (δ15N) to Measure Associative N2 Fixation
Both 15N isotope dilution and natural abundance methods depend upon differences in isotopic composition of the sources of N used for plant growth, i.e. atmospheric N, soil N and fertiliser N. Both methods require a non-N2-fixing reference plant and therefore it is essential that the reference plant and the test plant with associative N2 fixation have a similar root architecture and can extract N from the soil at the same rate in space and time [59]. Neither 15N method is suitable for quantification of the total amount of N2 fixation by free-living bacteria because of the difficulty of separating N2-fixing microorganisms from the soil for 15N analysis.
The 15N isotope dilution technique involves supplying a 15N enriched (or depleted) source of N to the soil so that it is significantly different from the natural abundance of the atmospheric N2 [59, 60]. For accurate measurement, the spatial and temporal availability of the isotope should be uniform [60]. The 15N isotope dilution method has been used to estimate N2 fixation associated with sugar cane, forage grasses, cereals and actinorhizal plants grown in soil, mostly in tropical systems.
The natural abundance (δ15N) method is exactly analogous to the isotope dilution method except that endogenous 15N in the soil is used [61, 62]. The natural abundance method has an advantage over isotope enrichment methods in natural ecosystems because disturbance of the system is unnecessary [62], but other factors can affect δ15N in plants, such as N from precipitation (NOx, NH3), the depths in the soil from which N is taken up and the form of soil N that is used (organic N, NH4+ or NO3-) [63]. The ability of the natural abundance method to measure associative N2 fixation depends on N2 fixed by associative microorganisms being predominantly taken up by the plant rather than going into the soil N pool [62]. Application of the 15N natural abundance technique in oil palms in the field in Brazil identified diazotrophs with a high potential for N2 fixation, but estimates of N2 fixation could not be calculated because of the absence of a suitable reference plant [64] highlighting a significant limitation of this method.
4.4. N Budget (N2 Fixed by Difference)
Giller and Merckx [23] suggested that the ultimate test of the contribution of N from fixation is to measure net inputs of N over long periods (>10 years) in the field, i.e. an N budget. However, this may be difficult as it requires measuring all inputs and outputs of N over this period, including inputs from fertilisers, wet N deposition, dry N deposition, run-on and uptake from lateral flow, outputs from crop/animal removal, gaseous losses, N leaching and soil erosion. A number of studies using long-term field experimental data have shown considerable N gains which were attributed to inputs from NS N2 fixation (for example [18, 22, 65-67]).
In the Rothamsted Broadbalk experiment, during the period from 1852-1967, Jenkinson [68] calculated that inputs from N2 fixation were between 18-28 kg N ha-1 year-1 in a plot that received no fertiliser, and 23-35 kg N ha-1 year-1 in a plot that received inorganic fertiliser without N. These estimates were obtained after adjusting for wet deposition from rainfall (5 kg N ha-1 year-1), dry N deposition (10 kg N ha-1 year-1) and for inputs in the seed (3 kg N ha-1 year-1) all of which were measured at some time during the course of the experiment [68]. Estimates indicate N deposition in rainfall and dust may range between 3-5 kg N ha-1 year-1 over much of Africa and Australia, and 10-50 kg N ha-1 year-1 in more densely populated areas such as in Europe [23, 69].
To achieve a reliable N balance it is necessary to have a very high repeatability and accuracy of N measurements through strict sampling protocols and extremely high sample numbers to enable the mean soil N to be precise enough to determine statistically significant changes in soil N [70, 71]. From a scientific viewpoint, N balance studies only give an indirect measure of N gains due to NS N2 fixation which can be useful for supporting other more direct measures. However, from a grower’s perspective, statistically significant measures of N accumulation provide valuable information for planning N fertiliser inputs for a crop.
4.5. Use of Multiple Techniques and New Methods
Used in conjunction with other measures such as the C2H2 reduction assay, N budgets may increase the certainty of estimates. For example, Shearman et al. [72] found that in grass pastures inoculated with a range of known N2-fixing bacteria, rates of C2H2 reduction were strongly correlated (r=0.92) with N accumulation measured by the Kjeldahl method. 15N aided N balance studies have been used to strengthen evidence for associative biological N2 fixation in sugar cane [65, 73, 74], where there was good agreement between estimates of biological N2 fixation from N balance and isotope dilution. However, the authors were careful not to assume the same rates of fixation occurred in the field because the conditions of the experiment differed from those in the field [73].
Much of the information on estimates of N2 fixation using techniques that are currently available apply to one instant in space and time or over a short period of assay [58]. However, knowledge of the conditions that favour N2 fixation and the rates at which fixation responds to changes in environmental conditions can be used to obtain estimates for a wider region if environmental conditions in those regions are known (e.g. meteorological records; cropping statistics and soil maps). Gupta et al. [22] used this principle to derive estimates for parts of the southern agroecological zones of Australia. Using information from other studies on the effects of different soil moistures, temperatures and carbon sources, potential N2 fixation in different zones was determined using a spatial analytical tool (ArcviewGIS Spatial Analyst, v3.1). Use of this principle with a range of measurement strategies may provide useful information about regions that are most likely to benefit from NS N2 fixation and where new advances can be made.
New techniques using microarray technologies have the potential to simultaneously measure the dynamics and/or activities of most microbial populations in the complex soil environment [75, 76]. Zhang et al. [37], used a nifH-based short oligonucleotide microarray and showed that this technique allows quantification and mapping of the abundance, diversity and activities of N2-fixing populations. This approach is likely to assist in the identification of regions and managements that favour inputs of N from NS N2 fixation. With the availability of complete genomes for several diazotrophic rhizobacteria and our increased ability to conduct in-depth genomic and functional analysis of candidate genes, we can now interrogate the specific features of diazotrophic endophytes. Such information on the molecular mechanisms of NS N2 fixation should enable the development of agronomic options to improve N2 fixation in non-leguminous crops [77].
5. FACTORS AFFECTING NS N2 FIXATION
5.1. Soil and Environmental Factors
Edaphic, environmental and management factors have a significant impact on the composition of diazotrophic communities and potentially their function [41,78, 79]. Varietal-based differences in the diversity of diazotrophic communities have been reported for wheat, barley, rice, sorghum [80-82] and perennial grasses [41]. Differences in soil environments at the micro-scale can also influence the composition of diazotrophic communities [83].
Nitrogenase proteins are extremely sensitive to O2 and on exposure to air they are rapidly and irreversibly inactivated [84]. Therefore, NS N2-fixing bacteria need mechanisms to exclude O2 for N2 fixation (nitrogenase activity) to occur. Some bacteria, e.g. Azotobacter, Azomonas, Beijerinckia and Derxia exclude O2 through rapid respiration or the formation of extracellular polysaccharide [29, 85]. However, most culturable diazotrophic bacteria will only fix N2 under microaerophilic or anaerobic conditions [29]. Anaerobic conditions can be created by saturating soil moistures and substantial amounts of N2 fixation have been measured under these conditions [86]. In aerated soils, aggregate formation in the soil allows microaerophilic and anaerobic conditions to coexist simultaneously under aerobic conditions. Furthermore, substrates such as dissolved organic C can be allocated into both aerobic and anaerobic fractions and processes [87] and so, it is possible for soluble products from organic matter decomposed under aerobic conditions, to supply C energy to microaerophilic and anaerobic N2-fixing bacteria within aggregates.
The second major condition required for NS N2 fixation is the availability of C as an energy source. Free-living N2-fixing bacteria generally rely on decomposing plant material above and below ground from crops and pastures. Associative N2-fixing bacteria utilise root exudates within a rhizosphere association with plants and other organisms. In both environments, other microbial groups compete for limited energy resources. Endophytic N2-fixing bacteria, on the other hand, have ready access to C and nutrients from within the plant [46].
Crop residues contain cellulose and hemicellulose which comprise 50-70% of its dry weight [88]. A few species of N2-fixing bacteria (Azospirillum spp.) are able to use straw directly for fixation [89], but most N2-fixing bacteria rely on decomposition to smaller components by other organisms [90]. Almost all N2-fixing heterotrophic bacteria are able to utilise the products of cellulose decomposition including carbohydrates and some organic acids and alcohols [91, 92]. Rates of N2 fixation are proportional to the amount of crop residue available and to rates of decomposition [19]. The retention of crop residues can alter the composition of diazotrophic community structure, increase nifH gene abundance and N2 fixation [82, 93, 94]. Root exudates, e.g. carbon containing compounds and quorum-sensing compounds, have been shown to influence the composition and function of nifH communities in the rhizosphere, and N2 fixation rates of 4-20 kg N ha-1 year-1 were predicted by Jones et al. [95]. Gupta et al. [41] observed a plant-based selection of nifH communities in the root environments of different summer-active perennial grass species. They found that diversity of diazotrophic bacteria was significantly higher in the rhizosphere than in the roots and that both the rhizosphere and roots supported higher N2 fixation than in cropping soils during summer.
It is well known that inorganic mineral N in soil can inhibit N2 fixation by NS microorganisms [96]. However, the dynamics of N2-fixing microbial populations are linked to available C:N ratios. For example, when C is abundant, excess ammonium N can be assimilated by other microbial populations allowing N2 fixation to occur, but with low C, excess ammonium N concentrations inhibit N2-fixing populations [97]. In the presence of large amounts of crop residue with wide C:N ratios, decomposition can be slow. But the addition of N increases the rate of decomposition (making C available for use by N2-fixing bacteria [30, 90]. Other mineral nutrients may influence NS N2 fixation. Mo and Fe are components of the nitrogenase enzyme, but they are rarely limiting in natural environments [98]. On the other hand, applications of P can significantly increase NS N2 fixation in crops [99, 100] and in grasslands [101, 102] particularly in nutrient poor soils. Reed et al. [99] and Smith [100] concluded that the strong inverse relationship between N2 fixation and mineral N content in the soil is mitigated by the availability of P.
High soil water contents have been used to promote N2 fixation in soils by reducing O2 at the sites of fixation [86]. However, in unsaturated soils, it is important to maintain aggregate structure and O2 gradients. In disturbed soils in the laboratory, a minimum of 50% water holding capacity was required for nitrogenase activity [103], whereas in in situ assays in undisturbed soils in the field, nitrogenase activity occurred at soil water contents below 30% water holding capacity [19]. In some environments, N2-fixing bacteria have adapted to harsh semi-arid environments e.g. lichens (containing cyanobacteria or other free-living bacteria in association with a fungus) [104, 105] and rhizosheaths around the roots of perennial grass species, and can contribute significant amounts of biologically fixed N [106, 107]. Rhizosheaths support enriched organic materials, greater water contents and a higher density of microorganisms including associative diazotrophs [106] and up to 9 kg N ha-1 year-1 has been measured [108].
N2 fixation has been shown to occur in situ in temperature extremes from near 0oC in Antarctica [109, 110] and in the Arctic [111] to desert environments where N2-fixing bacteria utilise morning dew or summer rains [105] but must survive during intervening hot dry conditions up to 60oC [98]. Laboratory experiments indicated that the most favourable temperatures for N2 fixation were between 30 and 35oC, with a range from 4-45oC [103]. The variation for the best temperature range for activity may depend upon the organisms present and the climatic conditions at each environment [98, 103].
Soil characteristics can significantly alter the potential for NS N2 fixation. For example, nitrogenase activity by free-living bacteria extracted from soil is best at pH 7-7.5 regardless of the pH of the original soil [112], and liming can increase the abundance of nifH-containing rhizobacteria in acidic soils [94]. Clays are highly reactive colloidal particles that interact strongly with microorganisms [113]. Roper and Smith [112] observed that the presence of montmorillonite clay increased N2 fixation by free-living bacteria. Clays increase macroaggregate formation [114] which creates sites of low O2 concentration [115] thus favouring N2 fixation. Microsites within intra-aggregate pore spaces and interior parts of aggregates not only provide suitable environments for nitrogenase activity but also protect bacteria from environmental extremes [116]. Although halophilic bacteria (e.g. Halomonas maura) isolated from saline soils have been shown to contain nifH genes and fix N2 [117], there is little other information on the impact of salt on NS N2-fixing bacteria in terms of growth and N2 fixation [118] particularly in agricultural soils.
Heavy metal (Zn, Cu, Ni, Cd, Cr, Pb, Hg, As) contamination can reduce abundance of NS N2-fixing bacteria [119, 120] and N2-fixing activity [121]. However, some strains of Azospirillum brasilense showed adaptation to heavy metals (Co, Cu and Zn [122]). The response of associative N2 fixation to heavy metals seems dependent on the tolerance of the plants themselves to the contaminant [123]. For example, roots of aluminium-tolerant plants exude significantly higher amounts of low molecular weight dicarboxylic acids which not only chelate Al3+ protecting the plant, but are also C sources for Azospirillum spp. and other N2-fixing bacteria [36, 91].
5.2. Management Practices
Minimum tillage systems support the stability of the aggregates especially macroaggregates that are critical for the development and maintenance of microsites of reduced O2 tension and for protection against biocidal exposure [116]. Any increase in soil disturbance reduces aggregation, reduces soil C and disrupts the soil pore network by which soil organisms interact [124-126]. As a result of all these factors, NS N2 fixation under no-till is characteristically higher than in cultivated soils [126]. However, biological changes in NS N2 fixation in response to adopting reduced/no- tillage practices can be slow sometimes taking several years to develop [20, 116, 127].
In no-till systems, populations of soil macrofauna such as ants (and termites) and earthworms are generally more abundant [126]. Significant amounts of N2 fixation can occur in the guts of earthworms [128], termites [129, 130] and arthropods [131], e.g. 4-10 kg N ha-1 year-1 [131, 132]. Crop rotations can profoundly modify the soil environment by influencing the removal of nutrients from the soil, return of crop residues (including quality and quantity), development and distribution of bio-pores, and dynamics of microbial communities [133] and therefore, are likely to affect the potential for N2 fixation. Information on the impact of pesticides on NS N2 fixation is relatively sparse and the effects are mixed. Among the pesticides, herbicides appear to have least significant effects on soil organisms, whereas insecticides and especially copper fungicides can be quite toxic [134]. Fungicides (methyl N-(1H-benzimidazo-2yl) carbamate and tetramethylthiuram disulfide) [135] and the herbicide glyphosate [136] have been shown to have negative impacts on the abundance of diazotrophs, whereas other studies indicated stimulation of populations and activities of N2-fixing bacteria, e.g. with the insecticide (hexachlorcyclohexane) [137] and a range of herbicides [138].
Associative N2 fixation has been suggested to be under the genetic control of the host plant [12, 139]. Differences in associative N2 fixation have been observed between different lines of rice [140], wheat [82, 141], maize and sorghum [142, 143], millet [144], and among various species of grasses [41, 145] and weeds [146]. Characteristics which contribute to high N2-fixing genotypes include a reduced transpiration rate, lower numbers of stomata and increased root exudates with a high concentration of dicarboxylic acids [143, 147]. Wood et al. [148] suggested that plants with an increased release of photosynthate to the rhizosphere should be a priority for the future development of broad-acre agricultural systems that are more self-sufficient for N nutrition.
6. TRANSFER OF FIXED N FROM DIAZOTROPHS TO PLANTS AND OTHER ORGANISMS
The transfer of N fixed to plants is likely to depend on the location at which N2 fixation occurs. Endophytic diazotrophs can supply biologically fixed N directly to the host [149], e.g. N2 fixation by endophytic bacteria associated with sugarcane can directly contribute more than half the crop’s N requirement [65, 150]. Because endophytic diazotrophs have only been observed in intercellular spaces, vascular tissue, aerenchyma and dead cells and not within living host cells, James [45] suggested that N transfer from these organisms is likely to be dependent on their death and release of fixed N. Transfer to plants of N fixed by diazotrophs or N contained in non-fixing microbial biomass in the soil or rhizosphere is also likely to be dependent on the death of these bacteria and release of ammonium or amino acids [151, 152], although excretion of nitrogenous substances during bacterial growth can also occur (e.g. Beijerinckia derxii [153]; cyanobacteria [154]).
In rhizosphere associations, N fixed can either be directly taken up by the plant or remain in the surrounding soil N pool (Fig. 1). There is little information about the proportions of N transfer to each of these pools. However, transfer of fixed N to plants from associative N2-fixing bacteria has been demonstrated using 15N2 by Giller et al. [55, 155] and others reviewed by Boddey [156] and James [45]. Release of N following the death of diazotrophic bacteria in the rhizosphere can be rapid due to wetting and drying cycles and microbial predation.
7. ENHANCING THE VALUE OF NS N2 FIXATION – A WAY FORWARD
Kennedy and Islam [17] expressed an optimism that up to half the N requirements of some cereal crops might be met from NS N2 fixation in the future through the use of genetic tools and inoculant biofertilisers. In addition, Beatty and Good [6] proposed two other strategies (1) developing root nodule symbioses in important cereal crops such as wheat, rice and maize and (2) introducing nitrogenase genes into a plant organelle.
7.1. Inoculation
There have been many studies on inoculation with N2-fixing bacteria of non-legumes (predominantly cereals and grasses), with reported above- and below-ground increases in total plant growth and N content [157]. The most successful inoculation responses have been in pot trials under controlled conditions (e.g. [158-161], but inoculation experiments in the field have been less consistent [162]. Andrews et al. [163] concluded that currently no NS N2-fixing bacterial inoculant is available that can match the consistency of N fertilisers for reducing soil N deficiencies.
One of the difficulties of inoculating soils with bacteria is that the inoculants generally decline rapidly due to competition with the native microflora [164, 165]. Inoculants compete with other microflora for available nutrients or become food for indigenous micro- and macro-fauna [166]. Hence the ultimate test for even the most effective beneficial organism is the ability to survive and colonise plant roots in the presence of much larger populations of indigenous microorganisms [157]. Inoculum formulation and application technology, e.g. along with organic matter (compost or peat) or micro-granulated inoculum, are likely to be crucial for inoculant survival and success [167].
7.1.1. Endophytes and GMOs
Endophytes are more likely to be successful inoculants because they can escape competition from indigenous microflora and can directly access the required energy source from the plant. Increased success with endophytic N2-fixing inoculants may be possible through genetic manipulation. For example, An et al. [168] suggested that manipulation of the promoter of the nifA gene in a N2-fixing bacterium that has a high colonisation competence may achieve stable associative N2 fixation in cereals. A similar approach has been put forward by Bloemberg [169]. Other advances using molecular strategies may be possible, e.g. the creation of ammonium excreting mutant diazotrophs, in which the mechanisms by which ammonium inhibits N2 fixation, are disarmed [170] or the increased production of nitrogenase reductase such as in an Azospirillum brasilense mutant [171]. However, the survival of such mutants in the field is uncertain [45].
7.1.2. Inoculants With Dual Benefits
Greater benefits may be possible where inoculants have a dual benefit through increased N nutrition via N2 fixation coupled with the production of plant growth hormones. There are several groups of organisms that are known to fix N2, produce phytohormones and/or provide protection against fungal and bacterial pathogens [171-173]. Hafeez et al. [174] showed that amongst 17 rhizobacteria isolated from different ecological regions, 15 fixed N2 and all produced various concentrations of indole-3-acetic acid, and at least one of the isolates produced siderophores. Some actinobacteria (Microbacterium sp., Micromonospora sp. and Arthrobacter sp.) contain nifH genes and fix N2 [82, 175], but can also colonise cereals as endophytes where they promote plant growth via phytohormone production, and suppress multiple root pathogens [176].
7.1.3. Co-Cultures
Combinations of cellulolytic microorganisms and N2-fixing bacteria have been studied, mostly in controlled environments, to understand the synergy between each group of organisms. For example, Veal and Lynch [177, 178] found that mixed cultures of the cellulolytic fungus Trichoderma harzianum and the N2-fixing bacterium Clostridium butyricum co-operatively degraded cellulose and used the degradation products to fix N2 equivalent to 7.87 mg N fixed / g C lost. A similar rate (12 - 14.6 mg N fixed / g cellulose consumed) was measured with cellulose containing co-cultures of Cellulomonas gelida and Azospirillum lipoferum or A. brasilense or Bacillus macerans [179]. With wheat straw and the same organisms, these authors measured 17-19 mg N / g straw consumed, a value not dissimilar to that found by Lynch and Harper [180] (11.5 mg N / g straw lost) for a Penicillium corylophilum – Clostridium butyricum association. However, in co-cultures of a mutant strain of Cellulomonas sp. (strain CS1-17) and Azospirillum spp. with cereal straw, Halsall and Gibson [181] measured much larger rates of fixation (72 and 63 mg N / g straw utilised) which concurs with the theoretical upper limit of 75 mg / g straw calculated by Kennedy and Islam [17]. Halsall and Gibson [181] attributed these vastly increased rates to the efficiency of the mutant strain of Cellulomonas sp. (strain CS1-17) and to low background N levels in the experiment.
Co-culture inoculants of cellulolytic organisms and diazotrophs are unlikely to confer great benefits in the field because of the high diversity of cellulolytic organisms that occur naturally in the soil (e.g. [182-184]). The exception to this might be soils that have not had a history of significant carbon inputs and cellulolytic populations are not well developed. Combining enhanced cellulolytic capability with nitrogenase activity in the same organism is likely to increase the efficiency of transfer of energy to N2 fixation, but so far efforts to achieve this have not been demonstrated.
7.1.4. Non-culturable Microorganisms
The finding that non-culturable bacteria, including members of Betaproteobacteria and Actinobacteria, may be dominant N2-fixing microorganisms [32, 34, 42, 140] requires the development of tools to culture them in order to develop effective inoculants. Advances in culturing technology (e.g. Janssen [185]) including sequence-directed isolation of novel bacteria offer new hope to identify functionally important bacteria suitable for specific crops and environments.
7.2. Management Combinations
Development of combinations of management practices should maximize NS N2 fixation. Cropping systems which combine high C inputs and good soil structure, e.g. conservation farming practices or perennial grass systems, are likely to be ideal. No-tillage practices combined with crop residue retention have increased rapidly world-wide in response to a range of pressures, and by 2003 for example, it was estimated that in Western Australia, 86% of farmers had adopted no-tillage [28]. Many of the field studies on NS N2 fixation in the past were done with soils where stubble retention had recently been adopted (e.g. [19]). Soil microbial composition and functions respond slowly to changed managements [116] and therefore it is likely that if similar experiments were conducted today on sites with long-term no-tillage and crop residue retention, different rates of N2 fixation might be found, particularly in areas under continuous cropping.
A system which supports 100% ground cover 100% of the time is likely to provide continuous inputs of C either by rhizodeposition or by inputs from above-ground residues. In ‘pasture-cropping systems’ where native summer-active perennial grasses are coupled with winter cereals it is proposed that biological N inputs are sufficient to supply the needs of the cereal crop. Using δ15N techniques, Mordelet et al. [186] and Abbadie et al. [187] were able to identify contributions from NS N2 fixation of up to 17 % of the annual savannah requirement for N. In pasture-cropping systems, fixed N is likely to be protected from leaching losses due to uptake by the grasses in autumn and later release by mineralisation from stubble and decomposing roots to a cereal crop during winter when the grass is not active [188]. Further research is needed to understand the N dynamics of pasture-cropping systems and to evaluate their potential in agriculture.
Little is known about the potential for N inputs via N2 fixation with other plants including weeds and other non-legume components of pastures except for a small study by Conklin and Biswas [146] who observed NS N2-fixing bacteria and nitrogenase activity (C2H2 reduction) associated with 20 weed species.
7.3. Plant Based Solutions
Only in Brazil are there varieties of sugar cane that have been shown to fix over 60% of their nitrogen (>150 kg N ha-1 year-1 [65]). Elsewhere in the world, measurements of contributions to N supply in sugar cane via biological N2 fixation have been small [189] although specific associations between diazotrophic bacteria and sugar cane have been observed [190]. It has been argued that this may be due to sugar crops in Brazil being systematically bred for high yields with low fertiliser inputs [65, 150]. Baldani et al. [150] suggested that such a breeding process with low fertiliser inputs has led to the development of (or preserved) an effective association between N2-fixing bacteria and the plant. Almost all of our modern crop varieties have been developed in conjunction with the use of nitrogen fertilisers suggesting that the capacity for significant associative N2 fixation may have been lost during breeding processes. Therefore, examination of the capacity for associative and endophytic N2 fixation in the wild relatives of wheat and other cereals, and the possibility of transferring this capability into modern varieties may have merit. Support for this notion can be seen with rice, for example Knauth et al. [140] examined the composition of diazotrophic communities associated with related rice cultivars (Oryza sativa) and wild species (Oryza brachyantha) and found that when grown under identical conditions in the same soil without N fertiliser there were remarkable differences in root associated nifH-gene expressing communities between the two cultivars. Furthermore, NifH fragments expressed in the wild species of rice roots indicated that the active diazotrophs were not related to cultured strains. In a separate study, Engelhard et al. [191] found that endophytic populations of diazotrophs differed with rice genotype and that the natural host range of the non-culturable Azoarcus spp. included rice, with wild and old rice varieties being preferred over modern cultivars. On the other hand, culturable species such as Azospirillum spp., Klebsiella sp., Sphingomonas paucimobilis, Burkholderia spp. were associated with more modern cultivars of Oryza sativa.
Evidence that ecosystems with low N promote NS N2 fixation occurs in a perennial grass (Molinia coerulea) which grows in oligotrophic environments. In another example, Gupta et al. [41] reported diazotrophic N2 fixation of 0.92 to 2.35 mg N / kg root / day with summer active perennial grasses such as Panicum species and Rhodes grass (Chloris gayana) in low organic matter soils of southern Australia. Hamelin et al. [34] observed that the rhizosphere of the perennial grass Molinia coerulea supported a diversity of N2-fixing bacteria, 56% of which contained NifH sequences that did not match any cultivated diazotrophs, but were dominant in the roots and surrounding soil. Further examination of such oligotrophic systems may yield diazotrophic communities that could be adapted to agricultural systems where they might increase the contribution from associative N2 fixation in agricultural crops.
SUMMARY
There is a range of estimates for NS N2 fixation in different cropping systems. A number of reviews suggest that significant amounts of N2 fixation (>30-40 kg N ha-1 year-1) are possible with C4 grasses including sugar cane in tropical regions (e.g. [12]), and where sugar cane has been bred with low N fertiliser inputs >150 kg N ha-1 year-1 has been measured [65]. Estimates in temperate and Mediterranean regions are less certain and range from 10-30 kg N ha-1 crop-1 [17, 18] to less than 5 kg N ha-1 year-1 [23], but it is likely that differences in methodology including application of individual methods have contributed to some of the reported variability. Environmental and management factors play an enormous role in the contribution of N from this beneficial microbial function. New technologies using molecular approaches, particularly when combined with isotope methods, are broadening our understanding of NS N2 fixation, and the molecular mechanisms of plant-diazotroph interactions. Microarray, pyrosequencing and Stable Isotope Probing (SIP) technologies offer an opportunity to investigate simultaneously both the diversity and function of diazotrophic microbial communities, and this may lead to the discovery of currently non-culturable bacteria that are functionally significant.
Generally there is a good understanding of the environmental factors controlling NS N2 fixation and this can be helpful in designing farming systems that promote N inputs from fixation, but most estimates of N2 fixation, particularly in the field were determined more than 20 years ago. Since then, farming practices have evolved towards intensive cropping (particularly with cereals), no-tillage and stubble retention, and further evaluation in terms of quantity of N fixed and identity of significant members of the N2-fixing community is needed. Similarly, alternative systems such as ‘pasture-cropping’ that benefit from N2 fixation associated with perennial grasses could be explored.
Further gains may be possible through inoculation with highly efficient N2-fixing bacteria particularly if they have the additional capacity to promote plant growth. However, the ultimate test for even the most beneficial inoculant is to be able to survive in soil and colonise plant roots. Inoculation with bacteria that can form an endophytic relationship within the plant (either in below-ground and/or above-ground parts) may increase the potential for success. However, many effective diazotrophic bacteria remain non-culturable and this may limit our ability to exploit them as inoculants unless new culturing techniques can be developed. New research using molecular techniques will reveal the true diversity of diazotrophic bacteria in agricultural and natural ecosystems and their potential to be used as inoculants in agricultural systems. Additionally, co-occurrence network analysis using nifH sequence data indicated the presence of complex co-occurrence patterns in the free-living diazotrophs than that known in symbiotic diazotrophs [202]. Such novel insights in to the ecology of diazotrophs may lead to development of inoculant mixtures that promote overall N2 fixation. Re-introduction into modern varieties of traits that promote the colonisation of highly efficient diazotrophic populations should further contribute biologically fixed N to agricultural systems, particularly in non-leguminous crops. Finally, NS N2 fixation provides an attractive option as an environmentally responsible alternative fertiliser source for sustainable food production, especially in lower organic matter and low fertility soils worldwide.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Financial support for the preparation of this review was provided by the Australian Grains Research and Development Corporation (GRDC) and from CSIRO. The authors thank Ramona Jongepier for assistance in preparation of the manuscript.


