All published articles of this journal are available on ScienceDirect.
Optimum Condition for the Formation of β-ionone by Thermal Decomposition of Carotenoids Extract from Orange Sweet Potato (Ipomoea Batatas L.)
Abstract
Introduction
The need for rose aromatic compounds is very high globally, where the beta-ionone compound as a precursor for rose aroma can be produced through the oxidation process of carotenoids, especially beta carotene. Therefore, Beta-ionone is the compound responsible for the rose fragrance, which is needed industrially. Carotenoids are easily degraded by various factors, namely heat, oxygen, light and enzymatic. This research has carried out heat degradation of carotene compounds extracted from orange sweet potato to determine the effect of heating time on forming aromatic compounds such as Beta-ionone and dihydroactinidiolide (dhA).
Methodology
Thermal degradation of carotene was carried out at 140°C for 1, 2, 3, and 4 h. The constant oxygen concentration of 7 l.h-1 was used to induce the oxidation. The spectrophotometric analysis of total carotene and the gas chromatographic-mass spectrometry analysis of volatile compounds were conducted to clarify the efficiency of the carotene degradation process and aromatic compounds' formation, respectively.
Results
Orange sweet potatoes contain 1.2% of carotenoids on a dry weight basis. Beta-ionone and dihydroactinidiolide compounds increased along with decreased total carotene value during heating. The results showed that heating sweet potato carotene at 140°C for 4 h caused carotene degradation of up to 93%. Therefore, the optimum thermal degradation treatment for the formation of β-ionone and dhA compounds was the heating condition at a temperature of 140°C for 4 h.
Conclusion
This research has shown the potential of sweet potatoes as a non-aromatic raw material that can be used to produce aromatic compounds. The use of the crude extract in this research means that the beta-ionone content in the product after oxidation is still low due to the presence of other components; this needs to be a concern for further research.
1. INTRODUCTION
Flavor compounds, including aroma-producing compounds, are essential to the development of the industry at this time [1, 2]. The presence of these aromatic compounds is generally obtained synthetically, although natural products have received high attention in the last decade. Natural products replace synthetic products due to their better traceability [2]. Especially for aromatic compounds used for products related to humans, such as food products or personal needs products, aromatic compounds will be associated with the human nervous system. Hence, the traceability of aromatic products becomes essential [3].
The aroma of roses is one of the aromas that the public likes [4]. The industrial demand for this fragrance is very high, but roses' availability has been limited recently. Therefore, chemical synthesis methods will be carried out more than the extraction process from roses to obtain the aroma of roses used for industry. Due to the high demand, it is necessary to find other alternatives to get the aroma of roses that can replace chemical synthesis methods [5]. Beta-ionone is the chemical compound responsible for producing the scent of roses. Since it is known that naturally occurring Beta-ionone is recommended for industrial use, the extraction process from roses is the recommended method. However, alternative approaches must be developed to obtain Beta-ionone naturally due to the limited availability of roses. In theory, Beta-ionone can be formed from the oxidation of carotenoid compounds. This oxidation process can occur naturally or intentionally [6]. Many studies have studied the oxidation process of carotene compounds to produce Beta-ionone [7-10].
Carotenoid compounds, especially Beta-carotene, are natural pigment compounds in vegetables and fruits such as palm, carrots, papaya, and orange sweet potatoes. These compounds are in substantial concentrations. The production of Beta-ionone from the oxidation of beta-carotene from crude palm oil and carrots has been carried out by several researchers before this. The oxidation process is carried out both thermally and enzymatically. There has yet to be a report on using the orange sweet potato as a raw material for beta-carotene compounds to produce Beta-ionone.
Orange sweet potato is one of the sources of high carotenoids in nature, with a content of 111 - 382 µg.g-1 depending on the variety of sweet potatoes [11]. Moreover, orange sweet potato is one of the commodities with very high productivity because this plant is straightforward to breed. Therefore, this plant will be able to provide the abundant raw material for Beta-carotene. This study will use a carotenoid extraction process from the orange sweet potato. The extract obtained will be thermally oxidized to see the amount of Beta-ionone produced. The beta-carotene oxidation process in producing beta-ionone in heat (thermal) conditions uses no chemicals.
In contrast, this process only uses heat conditions and a measured amount of oxygen. Optimization of the oxidation process and chemical changes from beta-carotene compounds during converting to Beta-ionone under thermal oxidation conditions are also observed. Therefore, this study could enhance the information related to technology development to produce rose-scented aromatic compounds without chemicals. The optimum condition of heat degradation of carotenoids from orange sweet potato could be the reference for practical guidance in transforming beta-carotene to beta-ionone.
2. MATERIALS AND METHODS
2.1. Study Area
The study was carried out from December 2021 to July 2023. The study was conducted at the Phytonutrient Laboratory, Department of Agricultural Product Technology and Laboratory of Biotechnology, Faculty of Animal Science, Andalas University, Indonesia.
2.2. Samples Preparation
The orange sweet potato was purchased from a local farmer in Padang City, West Sumatera Province, Indonesia. Two conditions were used to prepare raw materials: the preparation of orange sweet potato with blanching and the absence of blanching. This condition was done because the color changes from orange sweet potatoes, which quickly become brownish before drying. The orange sweet potatoes sorted, cleaned, and peeled were grated into smaller pieces using a cheese grater, weighed, and then blanched in hot water at 85°C for 1 minute. The grated sweet potato was then dried in an oven for 20 hours at 55°C. The dried sweet potatoes were weighed and mashed using a home kitchen blender. Finally, the powder was sieved using a 40 mesh.
2.3. Carotenoids Extraction
Approx. of 200 mL hexane was poured into 100 g of dried sample and homogenized. The mixture is then allowed to stand for the maceration process. The extraction process was induced by ultrasonic waves for 4 hours and continued for 8 hours without ultrasonic waves. The supernatant was collected by filtration. All these processes were repeated until all carotenoids had been extracted. The carotenoid extract was concentrated by evaporating the solvent.
2.4. Thermal Degradation of Crude Carotenoid Extract
The carotenoid was degraded using an oxidation apparatus, Metrohm Rancimat 743 (Herisau, Switzerland). The temperature was set at 140 °C with a 7 L.h-1 air flow rate into a 50 mL reaction tube that contained 4,5 g of glycerol. A 0,5 g of crude carotene extract was added and put in the Rancimat's heater block. The heating process was run for 4 hours, with hourly sampling intervals. Effluent air containing volatile organic acids, including volatile organic compounds from the crude carotene samples, was collected in a measuring vessel containing 10 mL of ethanol. A 2 mL of ethanol was filtered through a 0,2 µM polytetrafluoroethylene membrane filter before being injected into a Gas Chromatography-Mass Spectrometer.
2.5. Gas Chromatography-mass Spectrometer Analysis for Volatiles Detection
An apparatus of gas chromatography-mass spectrometer (Shimadzu GCMS-QP2010 Ultra, Kyoto, Japan) was used to evaluate volatile chemicals produced by the decomposition of crude carotene. Helium is utilized as a carrier gas. The DB-5 MS capillary column (30 m length, 0.25 mm inner diameter, 0.20 m film thickness, Agilent, USA) was used for chemical separation. The injector temperature was set at 220°C, the column oven temperature was raised from 60 °C to 260 °C, and the detector interface temperature was set at 270 °C. The temperature gradient program started at 60 °C and was constant for 4.5 minutes. The temperature was subsequently increased to 130 °C with 5 degrees in one-minute inclination and finally to 260 °C with 10 degrees in one-minute inclination. This temperature was kept for 2 minutes. With splitless injection mode, 1 mL of sample was injected into the GC-MS. The retention indices were compared using a mass spectral library search (Wiley 8 Library) and a peak area normalization approach to identify chemicals. The experiment's results were confirmed by repeating it at least three times [12, 13].
2.6. Analysis of Total Carotenoid by Spectrophotometer
A total of 50 mg of sample and 25 mL of hexane were added to a 25 mL volumetric flask, shaken until entirely homogeneous, and diluted with a dilution of 10 times. 1 mL of the solution was put into 9 mL of hexane in the test tube. The absorbance (A) of the solution was measured at a wavelength of 446 nm. Shimadzu UV-1800 was used for this measurement [5]. The total carotenoids in the samples were calculated by using the equation below:
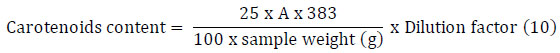 |
2.7. The Statistical Analysis
The analysis was executed using version 11.5 of the SPSS package program. Duncan's multiple ranges Post hoc test was applied following a one-way analysis of variance (ANOVA) data assessment. The mean ± SD of the triplicate samples was used to express the results. P<0.05 was used as the significance level for differences.
3. RESULTS AND DISCUSSION
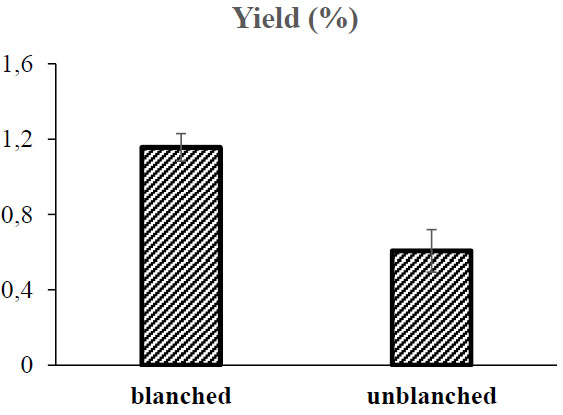
The orange sweet potatoes have been recognized as agricultural commodities that contain high amounts of phenolics. It indicated the browning symptom when the sample is peeled [14]. Therefore, the blanching treatment was applied to the sweet orange potatoes before they were grated in this study. Fig. (1) indicates the level of carotenoids extracted from the dry orange sweet potato powder, treated with blanching treatment and no treatment. The content of total carotenoids extracted from the blanched sample was higher than that extracted from the unblanched sample. The blanching treatment could enhance the extraction process of carotenoids from the orange sweet potatoes. The extraction of soluble starch could have also occurred during the blanching process despite the inactivation of Polyphenol oxidase. The loss of soluble starch in the cell membrane would affect the membrane permeability, which became low [15]. This condition released more carotenoids due to extraction.
The information that can be provided from the results of this blanching is the process optimization of carotenoid compounds in orange sweet potato samples. In the preliminary research carried out (data not shown), the process of peeling orange sweet potatoes took quite a long time, so the waiting time for the drying process would also be extended. Often, a browning process occurs due to enzymatic oxidation. This condition is the basis for the need to carry out the blanching process before drying. The resulting carotenoid yield also showed positive results where samples that underwent the previous blanching process could release more carotenoids than those not blanched after the solvent extraction process.
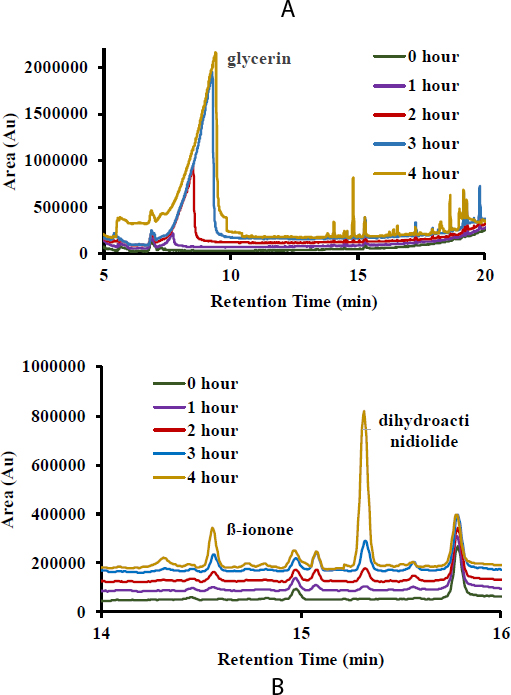
The results of the main objective of this study are demonstrated in Fig. (2). Fig. (2A) shows the GC-MS chromatogram of volatile compounds released from the degradation of carotenoids in the shelter solvent of ethanol. Many peaks were produced due to the heat oxidation of carotenoids. However, some in the blank sample (ethanol) also observed some peaks. Therefore, identifying produced compounds related to the degradation of carotenoids should be subtracted from the compounds in the blank. Based on the more profound observation (Fig. 2B), it can be identified that four compounds significantly increased according to the increase of the degradation time. The compounds were glycerin, Beta-ionone, dihydroactinidiolide, 1-hexadecene, and 1-heptadecanol with retention times of 8,9 minutes, 14,1 minutes, 14.85 minutes, 15.1 minutes, and 17,30 minutes, respectively. The presence of glycerin was confirmed not to come from the degradation of carotenoids because glycerin is a filler solution.
In this study, carotenoids were available in crude extract form. Carotenoid extracts have been mixed with other non-polar fractions. The large number of fatty acid components has shown this condition. The carotenoid extraction process with hexane solvent will cause all lipid fractions to be extracted; of course, this will affect the further oxidation process to produce beta-ionone. Previous research has carried out many oxidation processes of pure carotenoids, but the purification process is complicated to carry out in practice. Therefore, this research's oxidation process using crude carotenoid extracts will provide another perspective for producing beta-ionone compounds for downstream research results.
Moreover, beta-ionone and dihydroactinidiolide are theoretically related to carotenoid degradation from sweet orange potatoes. Meanwhile, 1-hexadecene may be produced for the continuous breakdown of non-polar compounds due to excessive heat. Beta-ionone and dihydroactinidiolide are recognized as the derived compounds of oxidized carotenoids [5, 16]. Beta-ionone is the main compound subjected in this study as the chemical compound responsible for the rose fragrance, while dihydroactinidiolide is also desired. Dihydroactinidiolide contains a carbonyl group that can react with nucleophilic structures in macromolecules, giving this compound a high reactivity potential, which might be used for medicinal properties. Both of these compounds are related to the aromatic compound that is usable industrially. Therefore, this result provides proper information regarding the potentiality of orange sweet potatoes as the source of Beta-ionone and dihydroactinidiolide.
Table 1 indicates the area of GCMS-detected compounds as the products that occurred after the degradation process of carotenoids in numerical values. The formation of beta-ionone and dihydroactinidiolide starts in the first hour of the degradation process. The dihydroactinidiolide formation is more significant than Beta-ionone formation. These two compounds are hypothesized to be substances derived from the carotenoid degradation process. Furthermore, glycerin was also detected. This condition occurred from the evaporation of glycerin due to the heating process. The longer the heating, the more glycerin will evaporate. Furthermore, 1-hexadecene and 1-heptadecanol were also detected, where these two compounds are substances produced from the fatty acid degradation process [16]. As is known, the crude carotene extract used in this research may still contain oil fractions because they are carried away during the extraction process.
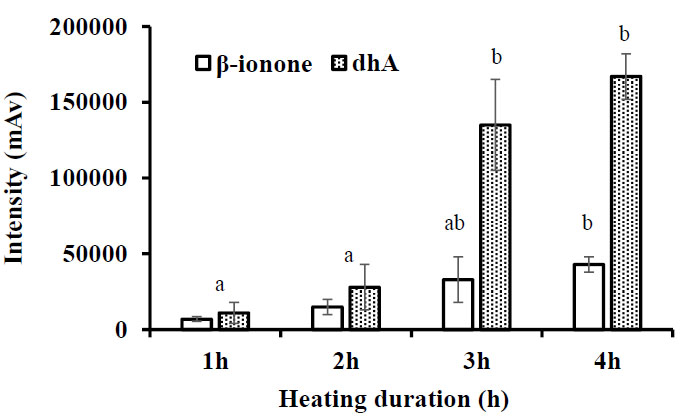
Syukri et al., 2022 [5] proposed the optimum condition for carotenoid degradation extracted from carrots. Instead of degradation time and oxygen concentration, temperature optimization has also been carried out from 100°C to 160°C with degradation time from 0 to 4 hours. The optimum temperature was 140°C with a 4-hour degradation process. A too high temperature will cause the oxidation process to occur at a very extreme rate, causing beta-ionone and dihydroactinidiolide to undergo further oxidation so that the levels become very low. Meanwhile, with a low oxidation temperature, the conversion process of beta-carotene to beta-ionone was prolonged and took a long time. In this study, with the sample of carotenoid extracted from orange sweet potato, the degradation temperature was set at 140°C, and it was found that the optimum degradation period was also achieved at 4 hours. This condition was similar to the obtained result in this study. Although the raw material was different, the characteristics of its extracted carotenoids might be similar. Fig. (3) indicates the changes in Beta-ionone and dihydroactinidiolide intensity during 4 hours of carotenoid degradation. The production of Beta-ionone and dihydroactinidiolide was not significant at the early time of degradation. After 2 hours of degradation, the formation of beta-ionone and dihydroactinidiolide had not yet happened. However, after 3 hours of degradation, significant beta-ionone and dihydroactinidiolide accumulated, while after 4 hours, only a tiny increment of these compounds was exhibited. From 2 hours degradation to 3 hours degradation, there was more than a five times increase of dihydroactinidiolide while Beta-ionone increased only about three times fold. This data indicates that the accumulation of dihydroactinidiolide was predominantly compared to Beta-ionone in this study.
According to Hamid et al. [17] and Sommerburg et al. [18], the formation of dihydroactinidiolide was a continuous reaction of the carotenoid oxidation process. This condition occurs due to excessive heat during the degradation process. Although dihydroactinidiolide is known as a natural flavoring that can be used in food and beverages since this study aimed to produce Beta-ionone, the presence of dihydroactinidiolide in high amounts can be upsetting. Further research is needed to discover controlled carotenoid oxidation conditions to produce Beta-ionone maximally.
Table 2 indicates the degradation rate of carotenoids after 3 and 4 hours of degradation. Following the formation trend of beta-ionone and dihydroactinidiolide, the degradation of carotenoids after 3 hours reached 91% and increased slightly after 4 hours to 93%. This data strengthens the previous data that indicated the significant formation of Beta-ionone and dihydroactinidiolide or carotenoid breakdown occurred after 3 hours of the process with the condition of 140°C temperature with 7 L.h-1 oxygen. Based on these results, the excess degradation time of more than 3 hours did not significantly impact the formation of targeted volatile compounds. Therefore, the adequate heat degradation time can be proposed for 3 hours.
This study has shown the potential for using orange sweet potatoes as a source of carotenoids, which can be converted into beta-ionone and dihydroactinidiolide. Previous research has also demonstrated the potential of carrots for the same condition. However, the use of sweet orange potatoes appeals due to their low price. Apart from that, the by-product of the extraction process of carotenoid using orange sweet potato as raw material is that starch can still be obtained, which cannot be extracted with hexane. Despite further research regarding the starch characteristics of orange sweet potatoes obtained after the carotenoid extraction process, the remaining starch can still produce other derivative products. This condition could be a topic for further research. In addition, it is challenging from the perspective of the aromatic compounds formed; the isolation or fractionation process of beta-ionone and dihydroactinidiolide needs to be carried out. Beta-ionone purity is needed at a high concentration as a precursor to the aroma of roses. Meanwhile, the oxidation results in this research are known to contain relatively high percentages of other components. Several things can be done next, such as a saponification process on the crude carotenoid extract and a fractionation process on the oxidation product.
| Treatments | (Area) | ||||
|---|---|---|---|---|---|
| Glycerin | β-ionone | dhA | 1-hexadecene | 1-heptadecanol | |
| 0h | - | - | - | - | - |
| 1h | - | 7142 a | 11213 a | 35618 a | 49281 a |
| 2h | 30129 a | 15012 b | 28215 b | 78120 b | 51021 b |
| 3h | 396021 b | 32998 c | 135163 c | 40912 c | 51027 c |
| 4h | 1092290 c | 43139 d | 167271 d | 38291 d | 50281 d |
| Treatments | Total Caretonoids Content (%) | Percentage of Degration (%) |
|---|---|---|
| No heating | 22,549±0,048 a | 0 |
| 3 hours | 1,872±0,005 b | 91.7 |
| 4 hours | 1,571±0,019 c | 93.03 |
Research related to the formation of beta-ionone from carotenoids has enormous industrial potential. Many materials contain high levels of carotenoids but currently have low economic value. The industry that can utilize this technology is the Crude Palm Oil (CPO) processing industry, where many carotenoids that have been wasted due to the bleaching process can be used to make more economically valuable products [19, 20]. Around 20-50% of carotene will be wasted when converting CPO into cooking oil, which can be used as raw material for beta-ionone production. This study needs to be focused on the future.
CONCLUSION
The use of orange sweet potato as a raw material source of carotenoids for producing valuable rose fragrances (beta-ionone compounds) has been proposed. Orange sweet potato carotenoid extract can be oxidized to beta-ionone at 140°C and oxygen at 7 L.h-1. The period for maximum Beta-ionone production ranges from 3 to 4 hours. The formation of dihydroactinidiolide from Beta-ionone occurs significantly due to uncontrolled oxidation processes after its formation. Moreover, the extract of carotenoid might contain any non-polar substance that might also oxidize with carotenoid. This condition caused the product of oxidation to vary. Therefore, further research needs to be carried out to overcome these conditions. The effort that can be further considered is the purification of carotenoid extract prior to oxidation. The simple purification technique that can be conducted is saponification of the extract since the impurities might be oil or fatty acid. Overall, this research has shown the potential for using orange sweet potato as a raw material to produce beta-ionone. In Indonesia, sweet orange potatoes are cheap and easy to get. Innovations from this research will be able to increase the economic value of orange sweet potatoes in the future. According to the research findings, there is great potential for converting carotenoids into beta-ionone, which will bring the primary objective of producing beta-ionone from natural sources closer to reality.
LIST OF ABBREVIATIONS
| dhA | = Dihydroactinidiolide |
| GC-MS | = Gas Chromatography Mass Spectrometer |
| ANOVA | = Analysis of variance |
| µg | = Micro gram |
| g | = Gram |
| mL | = Millilitre |
| L | = Litre |
| H | = Hour |
| µM | = Micro-Molar |
| C | = Celsius |
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [D.S], on special request.


