All published articles of this journal are available on ScienceDirect.
Genetic Diversity of Salvia Officinalis L. (Lamiaceae) and its Related Species using TU-DAMD Analysis
Abstract
Background:
Salvia tomentosa Mill., Salvia fruticosa Mill., and Salvia officinalis L. are Mediterranean species with different pharmaceutical and medicinal applications. However, genetic relationships among these species are still unclear.
Objective:
The study aimed to investigate the genetic polymorphism among S. officinalis L. (SO) and its related species S. tomentosa Mill. (ST) and S. fruticosa Mill. (SF) collected from different geographical regions in Syria.
Methods:
Touch-up directed amplification of minisatellite DNA (TU-DAMD) assay has been employed to assess genetic relationships among the studied Salvia species based on the estimated percent disagreement values (PDV).
Results:
Seventeen DAMD primers highlighted a mean of 90.419, 0.254, and 2.398% for polymorphism level (P%), polymorphic information content (PIC), and marker index (MI) values, respectively, across the three studied Salvia species. Unweighted Pair Group Mean Arithmetic average (UPGMA) analysis revealed that the studied Salvia samples were clustered into three main clusters; each species was split into one cluster. Overall, moderate P% of 72.662 and 70.374% was recorded for SO and ST species, respectively. Whereas, low P% of 51.429% was recorded for SF species.
Conclusion:
TU-DAMD marker is a potential tool for studying genetic relationships among the three studied Salvia species.
1. INTRODUCTION
Salvia is one of the largest plant genera. This genus includes approximately 1000 species [1] and belongs to the Lamiaceae family. Around 250 species of this genus are common in the Mediterranean regions, and the Salvia officinalis group consists of 11 species [2]. Among these 11 species, S. officinalis L., S. tomentosa Mill., and S. fruticosa Mill. species are the most three prominent species in the Mediterranean region [3].
According to Mouterde [4], S. tomentosa Mill (Tomentose sage) is native to areas ranging from South-East Europe to Transcaucasia, including Albania, Bulgaria, East Aegean Is., Greece, Krym, Lebanon, Syria, Transcaucasus, Turkey, and Yugoslavia [5]. Whereas, S. fruticosa Mill. (Greek sage) is a native species of the East Mediterranean basin and distributed from Italy, Sicily, and Cyrenaica through the South Balkan Peninsula (Albania and Greece) to West Syria [2]. Wild populations of these two species are widespread in Lebanon and Syria [4]. Conversely, S. officinalis L. (Common sage or Dalmatian sage) is native to the northern coast of Mediterranean regions and grows under wild type in the calcareous mountains of northern and central Spain, southern France, and the western part of the Balkan Peninsula [2, 6], and also to the Middle East and Mediterranean areas [7].
Whereas, S. tomentosa Mill. and S. fruticosa Mill. are present as wild, endemic species in Syria; however, the occurrence of wild S. officinalis L. among the Syrian flora is not mentioned by Mouterde [4].
Among the different Salvia species, Salvia officinalis L. displays the most valuable importance as an ornamental and medicinal plant. Due to its richness in bioactive compounds, it has a broad spectrum of uses ranging from food, pharmacology, and medicine to cosmetic applications [7-9]. It has been demonstrated that S. officinalis as a Mediterranean plant is characterized by exhibiting a high level of genetic diversity at the plastid genome (HT = 0.695) (as reported in other native aromatic/medicinal plants) and at nuclear DNA levels [8]. However, the genetic diversity in the canter of its origin is still unclear [3].
It has been demonstrated that some Salvia species have different pharmaceutical, medicinal, and industrial applications due to the richness of the essential oil in their bioactive components [7]. In Syria, they were used in folk medicine against winter diseases.
In Syria, S. tomentosa Mill. and S. fruticosa Mill. are known as miramiya, meramiya, mariamiya, and mirimiyah; they have a common Arabic name with a minor difference. S. officinalis is named cultivated mirimiyah, whereas S. fruticosa and S. tomentosa are known as wild mirimiyah. This classification is mainly based on their aromatic compounds. Mouterde [4] reported inferior aromatic compounds in S. fruticosa compared to S. officinalis. In Lebanon, S. fruticosa (syn. S. triloba L.fil. or S. libanotica Boiss. et Gaill) is an endemic species and named as mirimiyah or kas’in in Arabic. Indeed, this label was extended to Palestine and Jordan.
The molecular characterization of a given plant species is a potent tool for its conservation and breeding programs. DNA genetic variation within each Salvia species has been assessed in many reports. In this regard, generic polymorphism in S. officinalis L. has been assessed using random amplified polymorphic DNA (RAPD) markers [3, 10, 11]; single nucleotide polymorphism (SNP) and simple sequence repeat (SSR) markers [6, 9, 12, 13]; plastid DNA intergenic spacers [8]; inter simple sequence repeat (ISSR) markers [14], and recently, by amplified fragment length polymorphism (AFLP) markers [15]. As for S. fruticosa, RAPD markers [16] and microsatellites [17] have been used.
Different molecular marker systems have also been employed to study the genetic relationships within other Salvia species, for e.g., in S. hispanica L., by using the RAPD markers [18]; in S. lachnostachys, by using the ISSR markers [19], sequence-related amplified polymorphism (SRAP) and ISSR [20], and ISSR markers [21]; in S. lutescens var. intermedia, by using nuclear ribosomal DNA and plastid DNA sequences [22]; in S. divinorum, by using chloroplast simple sequence repeats (cpSSR's) [23]; in S. japonica, by using chloroplast and nuclear ribosomal DNA sequences and allozyme polymorphisms [24], and in S. euphratica sensu lato by using the internal transcribed spacer (ITS) and chloroplast DNA regions [trnT-trnL intergenic spacer (IGS)] markers [25].
Touch-down directed amplification of minisatellite DNA polymerase chain reaction (TD-DAMD-PCR) marker among various molecular markers available nowadays has been successfully employed for DNA genetic variability assessment in different plant crops, for e.g., in common bean landraces [26], Salvia species [27], Allium sp [28], carnation cultivars [29], commercial cotton [30], and S. tomentosa [31].
TU-DAMD assay has been recently employed to study the genetic diversity in Salvia judaica and Salvia palaestina [32], and more recently in Origanum syriacum L [33].
The genetic structure of plant populations reflects various interaction processes involving various phenomena (long-term evolutionary history of the species (shift in distribution, habitat fragmentation, and population isolation), mutations, genetic drifts, mating system, gene flow, and selection).
DNA genetic variability within S. officinalis group has not been investigated neither in Syria nor worldwide yet. In particular, studies relative to the Mediterranean S. officinalis L., S. tomentosa Mill., and S. fruticosa Mill. species are still lacking. Therefore, the current study has been conducted to assess the genetic relationships of S. officinalis and its related species using the TU-DAMD molecular marker.
2. MATERIALS AND METHODS
2.1. Sampling
Samples were collected from different geographical regions in Syria (Table 1). Cultivated accessions of S. officinalis L. (SO) (10 samples (2 from Lattakia, 2 from Jableh, 1 from Tartous, 2 from Hama, 2 from Damascus, and 1 from Darra), and natural populations of S. tomentosa Mill (ST) (5 samples (3 from Lattakia, 1 from Tartous, and 1 from Hama) and S. fruticosa Mill (SF) (4 samples (1 from Lattakia, 1 from Jableh, 1 from Banyas and 1 from Tartous) were collected from different location sites in Syria. Moreover, wild Origanum syriacum L. (Lamiaceae) species collected from Lattakia was used as the reference. Leaf sampling has been carried out during the blooming stage.
2.2. Genomic DNA Extraction
Leaf genomic DNA of the studied samples was isolated using CTAB (cetyltrimethylammonium bromide) as in the protocol described by Doyle and Doyle [34]. DNA fluorimeter instrument was used to determine DNA concentration. DNA was stored at –80°C until use.
Table 1.
| Species | Collection Site | Code | Altitude (m) | Annual Rainfall (mm) |
|---|---|---|---|---|
| S. officinalis | Lattakia | SOL1 | 20 | 800 |
| Lattakia | SOL2 | 60 | 800 | |
| Jableh | SOJ3 | 20 | 1200 | |
| Jableh | SOJ4 | 20 | 1200 | |
| Tartous | SOT5 | 247 | 1400 | |
| Hama | SOH6 | 170 | 750 | |
| Hama | SOH7 | 500 | 1500 | |
| Damascus | SOD8 | 950 | 260 | |
| Damascus | SOD9 | 800 | 300 | |
| Darra | SOD10 | 780 | 500 | |
| S. tomentosa | Lattakia | STL11 | 400 | 1100 |
| Lattakia | STL12 | 134 | 800 | |
| Lattakia | STL13 | 540 | 1100 | |
| Tartous | STT14 | 377 | 1500 | |
| Hama | STH15 | 300 | 1400 | |
| S. fruticosa | Lattakia | SFL16 | 650 | 110 |
| Jableh | SFJ17 | 75 | 1200 | |
| Banyas | SFB18 | 485 | 1400 | |
| Tartous | SFT19 | 890 | 1400 | |
| O. syriacum | Lattakia | OS | 80 | 800 |
| Primer No. | Primer Name | Primer Sequence 5'-3' |
|---|---|---|
| 1 | URP1F | ATCCAAGGTCCGAGACAACC |
| 2 | URP2R | CCCAGCAACTGATCGCACAC |
| 3 | URP9F | ATGTGTGCGATCAGTTGCTG |
| 4 | URP30F | GGACAAGAAGAGGATGTGGA |
| 5 | URP38F | AAGAGGCATTCTACCACCAC |
| 6 | OGRB01 | AGGGCTGGAGGAGGGC |
| 7 | FVIIex8 | ATGCACACACACAGG |
| 8 | HBV3 | GGTGAAGCACAGGTG |
| 9 | 14C2 | GGCAGGATTGAAGC |
| 10 | 33.6 | GGAGGTGGGCA |
| 11 | PM13 | GAGGGTGGCGGCTCT |
| 12 | HBVb | GGTGTAGAGAGAGGGGT |
| 13 | HVR | GGAGGTTTTCA |
| 14 | URP6R | GGCAAGCTGGTGGGAGGTAC |
| 15 | URP17R | AATGTGGGCAAGCTGGTGGT |
| 16 | M13 | GAGGGTGGCGGTTCCT |
| 17 | HVV | GGTGTAGAGAGGGGT |
2.3. TU-DAMD Assay
DNA genetic variability among the Mediterranean S. officinalis L., S. tomentosa Mill., and S. fruticosa Mill. species has been investigated using seventeen DAMD primers (Table 2). TU-DAMD assay was performed as more recently described by Saleh [32] in 25 μl total volume using a T-gradient thermal cycler (Bio-Rad, Hercules, USA) programmed as follows: 1 cycle for 4 min at 94 ºC, followed by ten cycles of pre-PCR involving 30 s at 94 °C for denaturation, 45 s at 55 °C for annealing, and 3 min at 72 °C for extension. The annealing temperature was increased by 0.5 °C/cycle for the first 10 cycles. Then, 30 cycles were carried out at a constant temperature of 55 °C as the annealing temperature, followed by a final extension at 72 °C for 10 min. Final PCR products were separated on a 2% ethidium bromide-stained agarose (Bio-Rad) in 0.5× Tris-borate-EDTA (TBE) buffer. Electrophoresis was carried out at 85 V for 2.5 h and visualized with a UV transilluminator. The molecular weight of TU-DAMD amplification products was estimated using a VC 100bp Plus DNA Ladder (Vivantis) standard.
2.4. TU-DAMD Data Analysis
Band scoring has been manually done as 0 or 1 for the absence or presence of each band size, respectively. The Unweighted Pair Group Mean Arithmetic average (UPGMA) analysis using the Statistica program [35] was constructed based on percent disagreement values (PDV). Genetic similarity among the three studied Salvia samples was determined [36]. Indeed, polymorphic information content (PIC) was determined [37] according to the following formula:
PIC = 1 ‒ Σ(Pij)2
Where, Pij is the frequency of the ith pattern revealed by the jth primer summed across all patterns revealed by the primers. Moreover, the marker index (MI) was also determined [38] according to the following formula:
MI = PIC × ηβ
Where, PIC is the mean PIC value, η is the number of bands, and β is the proportion of polymorphic information.
3. RESULTS
TU-DAMD markers produced PCR products with sizes ranging from 100-3000 bp. TU-DAMD polymorphism among the studied species yielded by OGRB01, 14C2, and M13 DAMD primers is shown in Fig. (1). The different primers produced a total band number ranging from 4 (HVR) to 19 (14C2) with a mean average of 9.824 bands/primer (Table 3). The number of polymorphic bands ranged between 2 (HVR) and 19 (14C2) with a mean average of 8.882 polymorphic bands/primer (Table 3). Six DAMD (URP2R, URP30F, FVIIex8, HBV3, 14C2, and M13) primers among the 17 DAMD-tested primers successfully produced a polymorphism level of 100%. Whereas, for the remaining primers, this value ranged between 50% (HVR) and 92.308% (HVV) (Table 3). Indeed, the PIC value ranged between 0.140 (URP9F) and 0.368 (M13) with a mean average of 0.254. As for MI, it ranged between 0.360 (HVR) and 6.175 (14C2) with a mean average of 2.398. In general, the TU-DAMD marker highlighted a mean average value of 90.419%, 0.254, and 2.398 for P%, PIC, and MI, respectively, across the three studied species (Table 3).
Genotypic-specific markers ranged between 0 (SOJ3) and 10 (SOH7) (Table 4). TU-DAMD assay highlighted 68 total genotypic-specific markers; they were 31, 19, and 18 for SO, ST, and SF species, respectively. Two DAMD primers (HVR and 33.6) did not reveal genotypic-specific markers among all the tested samples. Whereas, for the remaining 15 DAMD primers, this value varied from 1 (URP2R and M13) to 10 (HVV) with respect to genotypic-specific markers (Table 4).
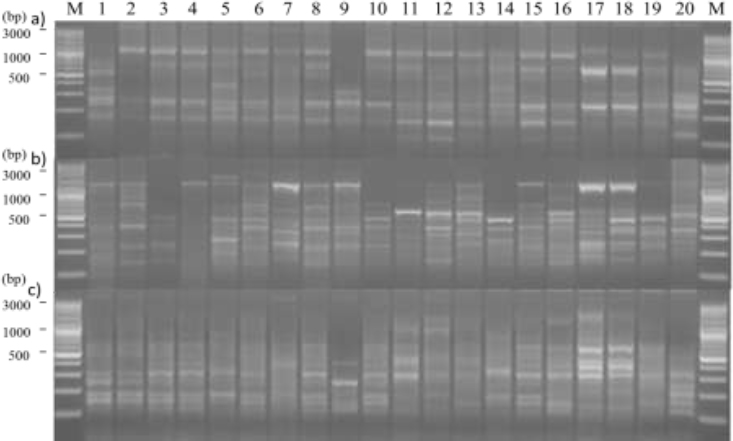
Table 3.
| Primer Name | TB | PB | P% | PIC | MI |
|---|---|---|---|---|---|
| URP1F | 11 | 10 | 90.909 | 0.249 | 2.490 |
| URP2R | 10 | 10 | 100.000 | 0.320 | 3.200 |
| URP9F | 5 | 3 | 60.000 | 0.140 | 0.420 |
| URP30F | 8 | 8 | 100.000 | 0.290 | 2.320 |
| URP38F | 11 | 10 | 90.909 | 0.246 | 2.460 |
| OGRB01 | 11 | 8 | 72.727 | 0.177 | 1.416 |
| FVIIex8 | 8 | 8 | 100.000 | 0.260 | 2.080 |
| HBV3 | 9 | 9 | 100.000 | 0.260 | 2.340 |
| 14C2 | 19 | 19 | 100.000 | 0.325 | 6.175 |
| 33.6 | 8 | 6 | 75.000 | 0.260 | 1.560 |
| PM13 | 11 | 10 | 90.909 | 0.368 | 3.680 |
| HBVb | 7 | 6 | 85.714 | 0.270 | 1.620 |
| HVR | 4 | 2 | 50.000 | 0.180 | 0.360 |
| URP6R | 7 | 6 | 85.714 | 0.210 | 1.260 |
| URP17R | 11 | 10 | 90.909 | 0.215 | 2.150 |
| M13 | 14 | 14 | 100.000 | 0.284 | 3.976 |
| HVV | 13 | 12 | 92.308 | 0.271 | 3.252 |
| Total | 167 | 151 | - | - | - |
| Average | 9.824 | 8.882 | 87.359 | 0.254 | 2.398 |
| Primer Name | SOL1 | SOL2 | SOJ3 | SOJ4 | SOT5 | SOH6 | SOH7 | SOD8 | SOD9 | SOD10 | STL11 | STL12 | STJ13 | STT14 | STH15 | SFL16 | SFJ17 | SFB18 | SFT19 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| URP1F | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| URP2R | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| URP9F | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| URP30F | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| URP38F | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
| OGRB01 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 7 |
| FVIIex8 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| HBV3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| 14C2 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 6 |
| 33.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PM13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HBVb | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| HVR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| URP6R | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| URP17R | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| M13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 7 |
| HVV | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 10 |
| Total | 4 | 5 | 0 | 1 | 5 | 2 | 10 | 1 | 2 | 1 | 6 | 8 | 3 | 1 | 1 | 3 | 6 | 5 | 4 | 68 |
| SO Total | 31 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| ST Total | 19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SF Total | 18 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
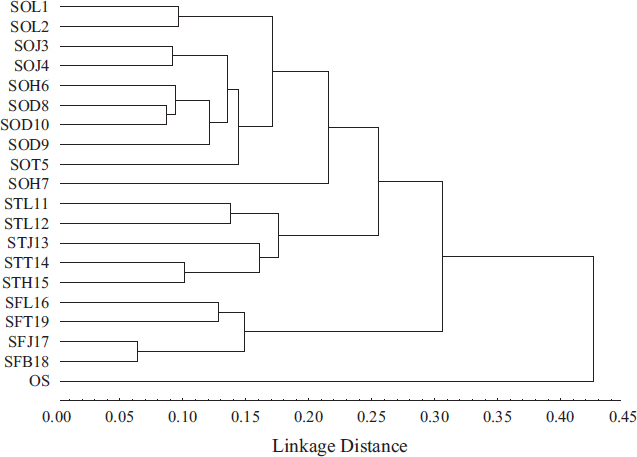
Clustering analysis has been conducted based on PDV (Fig. 2). This analysis showed that O. syriacum L. is genetically distant from the studied Salvia samples, as expected. More than this, it showed that Salvia samples are clustered into three main groups. The first cluster included SO samples of which SOT5 & SOH7 were genetically distinct from the remaining studied SO samples (PDV of 0.22 and similarity of 0.71) (Tables 5 and 6). Whereas, the second one included ST samples divided into two subclusters, with the first subcluster including STL11 and STL12 (PDV of 0.14 and similarity of 0.76) (Tables 5 and 6) and the second subcluster including STT14, STH15, and STL13. While, the third cluster included studied SF samples and divided itself into two subclusters; the first subcluster included SFL16 and SFT19 (PDV of 0.13 and similarity of 0.76), whereas the second one included SFJ17 and SFB18 (PDV of 0.06 and similarity of 0.91) (Tables 5 and 6). Tables 5 and 6 showed that the closest samples were SFJ17 and SFB18, exhibiting the lowest PDV of 0.06 and the highest similarity of 0.91. Whereas, the most distant samples were SOD8 and SFJ17 (PDV of 0.38 and similarity of 0.48) (Tables 5 and 6).
| Genotype | SOL1 | SOL2 | SOJ3 | SOJ4 | SOT5 | SOH6 | SOH7 | SOD8 | SOD9 | SOD10 | STL11 | STL12 | STJ13 | STT14 | STH15 | SFL16 | SFJ17 | SFB18 | SFT19 | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOL1 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOL2 | 0.10 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOJ3 | 0.14 | 0.11 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOJ4 | 0.16 | 0.13 | 0.09 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOT5 | 0.21 | 0.18 | 0.14 | 0.16 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOH6 | 0.18 | 0.12 | 0.09 | 0.13 | 0.11 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOH7 | 0.27 | 0.27 | 0.21 | 0.23 | 0.22 | 0.19 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOD8 | 0.22 | 0.18 | 0.13 | 0.15 | 0.17 | 0.10 | 0.19 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - |
| SOD9 | 0.21 | 0.19 | 0.17 | 0.17 | 0.17 | 0.13 | 0.16 | 0.11 | 0.00 | - | - | - | - | - | - | - | - | - | - | - |
| SOD10 | 0.20 | 0.15 | 0.10 | 0.14 | 0.12 | 0.09 | 0.21 | 0.09 | 0.12 | 0.00 | - | - | - | - | - | - | - | - | - | - |
| STL11 | 0.28 | 0.26 | 0.22 | 0.25 | 0.21 | 0.22 | 0.31 | 0.26 | 0.27 | 0.22 | 0.00 | - | - | - | - | - | - | - | - | - |
| STL12 | 0.30 | 0.28 | 0.25 | 0.26 | 0.24 | 0.24 | 0.32 | 0.30 | 0.26 | 0.26 | 0.14 | 0.00 | - | - | - | - | - | - | - | - |
| STJ13 | 0.27 | 0.25 | 0.22 | 0.22 | 0.23 | 0.24 | 0.34 | 0.26 | 0.26 | 0.22 | 0.18 | 0.16 | 0.00 | - | - | - | - | - | - | - |
| STT14 | 0.27 | 0.27 | 0.26 | 0.24 | 0.28 | 0.28 | 0.33 | 0.30 | 0.33 | 0.25 | 0.18 | 0.22 | 0.18 | 0.00 | - | - | - | - | - | - |
| STH15 | 0.23 | 0.22 | 0.17 | 0.19 | 0.21 | 0.21 | 0.28 | 0.24 | 0.28 | 0.20 | 0.12 | 0.19 | 0.14 | 0.10 | 0.00 | - | - | - | - | - |
| SFL16 | 0.30 | 0.32 | 0.28 | 0.28 | 0.30 | 0.29 | 0.35 | 0.31 | 0.32 | 0.28 | 0.25 | 0.29 | 0.24 | 0.24 | 0.20 | 0.00 | - | - | - | - |
| SFJ17 | 0.33 | 0.36 | 0.31 | 0.33 | 0.36 | 0.35 | 0.37 | 0.38 | 0.36 | 0.35 | 0.32 | 0.33 | 0.32 | 0.28 | 0.26 | 0.17 | 0.00 | - | - | - |
| SFB18 | 0.32 | 0.33 | 0.29 | 0.30 | 0.31 | 0.33 | 0.37 | 0.35 | 0.36 | 0.30 | 0.29 | 0.32 | 0.29 | 0.26 | 0.24 | 0.14 | 0.06 | 0.00 | - | - |
| SFT19 | 0.30 | 0.31 | 0.28 | 0.28 | 0.31 | 0.32 | 0.36 | 0.33 | 0.36 | 0.29 | 0.26 | 0.33 | 0.25 | 0.24 | 0.23 | 0.13 | 0.17 | 0.13 | 0.00 | - |
| OSL | 0.41 | 0.43 | 0.41 | 0.44 | 0.46 | 0.45 | 0.47 | 0.46 | 0.46 | 0.43 | 0.41 | 0.44 | 0.40 | 0.39 | 0.34 | 0.42 | 0.44 | 0.43 | 0.39 | 0.00 |
| Genotype | SOL1 | SOL2 | SOJ3 | SOJ4 | SOT5 | SOH6 | SOH7 | SOD8 | SOD9 | SOD10 | STL11 | STL12 | STJ13 | STT14 | STH15 | SFL16 | SFJ17 | SFB18 | SFT19 | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOL1 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOL2 | 0.84 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOJ3 | 0.78 | 0.83 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOJ4 | 0.75 | 0.79 | 0.86 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOT5 | 0.67 | 0.73 | 0.80 | 0.76 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOH6 | 0.72 | 0.82 | 0.87 | 0.81 | 0.84 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOH7 | 0.61 | 0.63 | 0.72 | 0.68 | 0.71 | 0.75 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SOD8 | 0.67 | 0.74 | 0.82 | 0.78 | 0.77 | 0.87 | 0.76 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - |
| SOD9 | 0.68 | 0.73 | 0.77 | 0.75 | 0.76 | 0.83 | 0.79 | 0.85 | 1.00 | - | - | - | - | - | - | - | - | - | - | - |
| SOD10 | 0.69 | 0.78 | 0.86 | 0.80 | 0.83 | 0.87 | 0.73 | 0.88 | 0.84 | 1.00 | - | - | - | - | - | - | - | - | - | - |
| STL11 | 0.50 | 0.56 | 0.63 | 0.57 | 0.66 | 0.66 | 0.53 | 0.61 | 0.58 | 0.64 | 1.00 | - | - | - | - | - | - | - | - | - |
| STL12 | 0.51 | 0.57 | 0.61 | 0.58 | 0.63 | 0.64 | 0.55 | 0.57 | 0.62 | 0.61 | 0.76 | 1.00 | - | - | - | - | - | - | - | - |
| STJ13 | 0.54 | 0.59 | 0.65 | 0.63 | 0.63 | 0.62 | 0.51 | 0.62 | 0.60 | 0.65 | 0.67 | 0.74 | 1.00 | - | - | - | - | - | - | - |
| STT14 | 0.51 | 0.52 | 0.56 | 0.57 | 0.53 | 0.53 | 0.50 | 0.53 | 0.46 | 0.59 | 0.65 | 0.60 | 0.66 | 1.00 | - | - | - | - | - | - |
| STH15 | 0.58 | 0.61 | 0.70 | 0.66 | 0.65 | 0.66 | 0.57 | 0.62 | 0.56 | 0.67 | 0.76 | 0.67 | 0.74 | 0.80 | 1.00 | - | - | - | - | - |
| SFL16 | 0.52 | 0.52 | 0.58 | 0.58 | 0.55 | 0.58 | 0.52 | 0.57 | 0.55 | 0.61 | 0.58 | 0.55 | 0.61 | 0.59 | 0.66 | 1.00 | - | - | - | - |
| SFJ17 | 0.49 | 0.46 | 0.55 | 0.51 | 0.49 | 0.51 | 0.51 | 0.48 | 0.51 | 0.51 | 0.49 | 0.51 | 0.50 | 0.52 | 0.58 | 0.76 | 1.00 | - | - | - |
| SFB18 | 0.49 | 0.48 | 0.56 | 0.53 | 0.53 | 0.52 | 0.49 | 0.50 | 0.49 | 0.56 | 0.51 | 0.50 | 0.53 | 0.55 | 0.59 | 0.79 | 0.91 | 1.00 | - | - |
| SFT19 | 0.48 | 0.49 | 0.54 | 0.52 | 0.50 | 0.50 | 0.47 | 0.51 | 0.45 | 0.54 | 0.52 | 0.45 | 0.55 | 0.54 | 0.57 | 0.79 | 0.74 | 0.79 | 1.00 | - |
| OSL | 0.33 | 0.33 | 0.37 | 0.30 | 0.31 | 0.34 | 0.34 | 0.34 | 0.33 | 0.36 | 0.30 | 0.29 | 0.34 | 0.30 | 0.40 | 0.35 | 0.34 | 0.33 | 0.34 | 1.00 |
4. DISCUSSION
DNA genetic variation among Salvia officinalis L. (SO), and its related species of S. tomentosa Mill. (ST) and S. fruticosa Mill. (SF), collected from different geographical regions in Syria, has been assessed using the TU-DAMD assay.
For S. officinalis, the application of the TU-DAMD marker showed a polymorphism level (P%) of 72.662% among the ten studied samples. Whereas, other reports revealed that this value for the same species varied between 32.03%-90%. Our data were compared with those reported by other investigations. In this regard, it was recorded to be 90% [14], 80.681% [3], 63.54% [13], 59.5% [11], 57.2% [10], and 32.03% [39], respectively. This difference could be attributed to the following factors: I. Studied population type and size, wherein the current study, samples were introduced, cultivated, and domesticated under different climatic conditions varied from dry to wet; while, for the other investigations, genetic diversity was carried out on natural populations. II. Marker system employed and primers number, wherein the current study, TU-DAMD marker was employed, whereas in the other investigations, RAPD [3, 10, 11] and ISSR [14, 39] have been employed. It has been demonstrated that species, geographical distribution, selection, and cross-pollination are considered the main factors affecting genetic diversity in Salvia species [40]. Genetic diversity observed in S. officinalis species in the current study compared to other ones has been summarized in Table 7.
Overall, the current study revealed P% to be 72.662, 70.374, and 51.429% for SO, ST, and SF species, respectively. This observation has been reported to be consistent with previous and recent published reports on the Salvia genetic diversity at the species level. Regarding this index, our data was found to be between 32.03% in S. officinalis [39] and 95.6% in S. lachnostachys [19]. reported in the related literature.
Table 7.
| Molecular Marker | Results | P% | References |
|---|---|---|---|
| 17 TU-DAMD | - | 72.66 | Current study |
| RAPD | 88 TB and 71 PB (ranged between 4-13 bands) with an average of 8.9 | 80.68 | [3] |
| 8 SSR | 165 total alleles (ranging from 13-30) and PIC ranging between 0.63-0.94, with an average of 0.81 | - | [9] |
| 39 RAPD | TB ranging between 2-13 bands and PB ranging between 0-12 bands | 57.20 | [10] |
| RAPD | - | 59.50 | [11] |
| 9 SSR | 125 TB (ranging between 8-21 loci) and PIC ranging between 0.70-0.92, with an average of 0.841 | 63.54 | [13] |
| ISSR | - | 90.00 | [14] |
| 4 AFLP PCs | - | 63.54 | [15] |
| 16 ISSR | 128 TB (ranging between 3-16 bands) and 41 PB (ranging between 1-5 bands) | 32.03 | [39] |
Echeverrigaray and Agostini [11] reported P% to be 59.5% within S. officinalis only and to be 73% when S. officinalis and S. sclarea were introduced together in genetic analysis, using the RAPD marker. Whereas, Boszrmenyi et al. [10] reported P% to be 57.2% in S. officinalis and 83.6% when S. judaica was introduced in the genetic analysis using the RAPD marker. Moreover, Mader et al. [12] reported genetic variability among 19 accessions of S. officinalis using SNP and SSR markers. They reported that the AMOVA test revealed 51% of the variance between the populations and 49% within the principal component analysis and that the samples were clustered in the main cluster. Whereas, Radosavljević et al. [6] reported a mean PIC of 0.802 in wild and cultivated populations of common sage S. officinalis L. using SSR loci. Indeed, Radosavljević et al. [13] reported a mean PIC of 0.841 in Salvia officinalis L. natural population using SSR loci.
Liber et al. [3] reported a P% of 80.681% using the RAPD marker among ten natural populations of S. officinalis. They reported that molecular variance analysis (AMOVA) showed most DNA genetic variation to be related to differences among the studied samples within populations, and also that genetic differences were recorded among the populations. Whereas, Rešetnik et al. [9] reported a PIC average of 0.81 within S. officinalis population using the SSR loci. They reported clear genetic DNA polymorphism between wild and cultivated S. officinalis populations restricted from one geographical region using SSR markers.
Moreover, Sarrou et al. [14] reported a P% of 90% in 7 S. officinalis populations using the ISSR marker. Whereas, Altindal [39] reported a P% of 32.03% among 8 S. officinalis samples using the ISSR marker. Recently, Jug-Dujaković1 et al. [15] reported a P% average of 63.54% in S. officinalis using the AFLP marker.
Previously, Skoula et al. [16] reported 155 total bands among 48 S. fruticosa clones using the RAPD marker. Whereas, Leontaritou et al. [17] reported high genetic diversity within the same species using the microsatellites marker.
Tychonievich and Warner [41] reported that spontaneous hybrids occurred either in the wild or in cultivated type due to intentional crosses between S. officinalis and S. lavandulifolia, and also between S. fruticosa and S. tomentosa.
Genetic diversity in other Salvia species has also been investigated. In this regard, Cahill [18] reported DNA genetic diversity in S. hispanica L. using the RAPD marker as higher among wild types compared to domesticated and commercial types. Whereas, Song et al. [20] reported high genetic similarity reflecting low genetic diversity in S. miltiorrhiza using SRAP and ISSR markers. Indeed, Zhang et al. [21] reported the high importance of DNA genetic diversity of S. miltiorrhiza using ISSR marker in plant breeding programs. Moreover, Saleh [31] reported a P% of 82.911% combined with 0.264 and 2.269 for PIC and MI average values, respectively, in S. tomentosa using the TD-DAMD analysis.
Recently, Saleh [32] reported low genetic diversity (P%) in S. judaica (40.45%) and S. palaestina (42.31%) species, whereas a high genetic diversity of 90.00% has been recorded between the two mentioned species using the TU-DAMD marker.
Based upon TU-DAMD data presented herein, the current study revealed moderate genetic diversity (P%) of 72.662 and 70.374% for SO and ST species, respectively. Whereas, low genetic diversity of 51.429% has been recorded for SF species. While high genetic diversity of 90.419% has been recorded across the three studied Salvia species.
Genetic diversity observed across the three studied Salvia species in the current study could be attributed to outcrossing process, as similarly reported for S. officinalis [8], or variation between wild or cultivated S. officinalis species [9], or to spontaneous hybrids occurring either in the wild or cultivated Salvia species [41]; it can also be due to an interspecific hybrid, as similarly reported in S. divinorum [23], or reproductive biology, gene flow, seed dispersal, and nature selection [19]. These events have encouraged efficient gene flow, leading finally to heterozygosity and genetic diversity expansion.
CONCLUSION
Genetic diversity across the three studied Salvia species (S. officinalis L., S. tomentosa Mill., and S. fruticosa Mill.) grown in different geographical regions in Syria has been investigated through the TU-DAMD marker. This marker successfully discriminates among the three studied Salvia species. Cluster analysis revealed that the three studied Salvia species were clustered in three main clusters, with each cluster associated separately with each species. Due to the recent success of the TU-DAMD marker for the assessment of the genetic diversity of S. judaica, S. palaestina, and O. syriacum L. species, it is worth noting to expand its employment in molecular studies in order to discover its effectiveness in genetic diversity assessment of other plants species. Over all, the current investigation could be considered as the first report highlighting the genetic relationships among the three prominent Salvia species grown in Mediterranean regions. The genetic diversity observed among the three studied Salvia species could be considered as a potential tool to be exploited in Salvia breeding programs. The current study provides a useful tool to be integrated with the genetic and phytochemical diversity of these species, providing a potential benefit to plant breeding programs.
LIST OF ABBREVIATIONS
| AFLP | = Amplified fragment length polymorphism |
| cpSSR's | = Chloroplast simple sequence repeats |
| CTAB | = Cetyltrimethylammonium bromide |
| DAMD | = Directed amplification of minisatellite-region DNA |
| ISSR | = Inter simple sequence repeats |
| MI | = Marker index |
| P% | = Percentage polymorphism |
| PB | = Polymorphic bands |
| PIC | = Polymorphic information content |
| PDV | = Percent disagreement values |
| RAPD | = Random amplified polymorphic DNA |
| SNP | = Single nucleotide polymorphism |
| SRAP | = Sequence-related amplified polymorphism |
| SSRs | = Simple sequence repeats |
| TB | = Total bands |
| TD-DAMD | = Touch-down directed amplification of minisatellite DNA |
| TU-DAMD | = Touch-up directed amplification of minisatellite DNA |
| UPGMA | = Unweighted pair group mean arithmetic average |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the author [B.S] upon request.
FUNDING
This study was supported by the Department of Molecular Biology and Biotechnology, Atomic Energy Commission of Syria, P.O. Box 6091, Damascus, Syria.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGEMENTS
The author thanks Dr. I. Othman (Director General of AECS) and Dr. N. Mirali (Head of Molecular Biology and Biotechnology Department in AECS) for their support, and also the Plant Biotechnology group for technical assistance.


