All published articles of this journal are available on ScienceDirect.
Silicon Reduces the Severity of Fusarium Infection on Young Wheat Parts In Vitro
Abstract
Background:
Although silicon (Si) has been referred as an essential element for controlling destructive diseases of wheat, available data are limited about enhancing adult wheat resistance against Fusarium causing head blight. Also, no reports seem to exist on the use of Si to reduce Fusarium seedling blight (FSB) on young wheat parts in vitro. Under in vitro conditions, Fusarium infection happened at the seedling stage cannot be called Fusarium head blight, because it is not a “head” disease, instead it could be called “FSB”.
Objective and Methods:
This research aimed to elucidate the bio-efficacy of soluble Si at 1.7 mM to increase wheat resistance to FSB measured by latent period (LP) of detached leaf inoculation, area under disease progress curve (AUDPC) of Petri-dish inoculation and coleoptile length reduction (CLR) of a coleoptile infection detected in vitro. Si treatments were applied to six bread and durum wheat cultivars of contrasting susceptibility to disease infected with four Fusarium species displaying a diverse pathogenicity.
Results:
Differences were observed on wheat detached leaves and seedlings in the resistance of Si-Fusarium-inoculated treatments relative to fungal-inoculated-controls, showing the beneficial role played by this element in decreasing head blight disease symptoms on young plant parts under in vitro conditions. In all wheat cultivars infected with different Fusarium species, the application of Si did increase host resistance measured in vitro; 1.7 mM Si resulted in significantly higher LP and lesser AUDPC and CLR compared with controls. More importantly, Si at 1.7 mM increased host resistance of susceptible to moderately susceptible cultivars measured by LP, AUDPC and CLR to the same level of resistance exhibited by a wheat cultivar high in quantitative resistance without Si.
Conclusion:
This is the first report presenting the utility of three distinct in vitro bio-assays to investigate the effect of Si in the wheat- FSB pathosystem. The application of silicon to complement host resistance to head blight appears to be an effective strategy for disease management in wheat.
1. INTRODUCTION
Wheat, involving bread (Triticum aestivum) and durum (T. durum), is the most consumed and second most-produced cereal on earth and is a staple food by providing calories and proteins for more than 36% of the world’s population [1]. Wheat is susceptible to various diseases that can cause economic damage. Fusarium head blight is a devastating disease afflicting wheat and other small-grain cereals (i.e., barley, oat, rye, and triticale) globally [2]. Head blight disease is attributable to a number of 17 pathogens with specific growth habitats which undergo mainly under the Fusarium genus; F. graminearum and F. culmorum are the predominant and most pathogenic causal agents in the disease complex [3, 4]. In addition to yield losses of up to 75% this disease is of primary concern because of the large amount accumulation of mycotoxins, especially in the trichothecene group including deoxynivalenol (DON), in infected grain [5]. DON contamination has raised serious health threats to humans and animals when exposure levels are high. Pathogen infection of wheat kernels, spikelets, or the full head occurs during anthesis making them appear water-soaked as they lose chlorophyll and become bleached as a result of DON release in the wheat head, especially under humid and warm conditions [6]. As toxins may act as pathogenicity factors, head blight concern is exacerbated by the recent shift in the pathogen populations worldwide towards greater toxin production, pathogen vigor, and diffusion of highly pathogenic Fusarium isolates [7].
Host resistance is considered an efficient and environmentally friendly approach to decreasing disease damage [5, 6]. Inoculation methods to assess Type I, resistance to initial infection, and Type II, resistance to the movement and spread of the fungi within the spike are conducted at anthesis in adult plants [7]; however, several disadvantages were reported [8]. So, alternative in vitro methods at the early development stage in resistance evaluations were validated [9-11]. Under in vitro conditions, Fusarium infection that happened at the seedling stage cannot be called Fusarium head blight, because it is not a “head” disease, instead, it could be called “Fusarium seedling blight, FSB”. As reported in previous and novel studies [9-14], latent period (LP) (time from inoculation to sporulation), area under disease progress curve (AUDPC), and coleoptile length reduction (CLR) have been regarded as the most important in vitro components for assessing quantitative resistance in the wheat–FSB system. Wheat cultivars having higher LP and less AUDPC and CLR are considered to be more resistant when faced with fungal isolates than cultivars having lesser LP and higher AUDPC and CLR [9-14]. As long as LP, AUDPC and CLR are in vitro indicators of mechanisms of Type I and Type II occurring in the adult wheat plants during disease infection; the measured changes of these reactions on young wheat parts can be considered to be largely the same as pathogenic responses in wheat plants grown in the field [9-14]. However, although control strategies for head blight have centered on the use of genetic resistance [5, 6], its use is limited due to (i) that this resistance is largely quantitatively inherited and limited by additive genetic effects that may also be environmentally specific and (ii) potential breakdown in wheat resistance due to pathogenicity shift of Fusarium populations [7]. Therefore, a practical alternative tool is to manage mineral nutrition in order to increase disease resistance in wheat to Fusarium infection.
For instance, silicon (Si) stands out among mineral elements for its capability to alleviate the destruction of wheat during abiotic and biotic stresses [15]. Taken into account that wheat is recognized as a Si absorber (its absorption ability from the soil reported 50-150 kg Si/ha) and accumulator (accumulating Si in concentration up to 20 g/kg of dry weight) [16], novel evidence proved that the Si absorption and deposition could be elucidated by the active transport mechanisms inherent to the roots and the shoots [15, 17]. To date, Si reduces the intensity of a number of destructive diseases of wheat, i.e., blast (Magnaporthe oryzae); powdery mildew (Blumeria graminis f. sp. tritici); septoria leaf blotch (Zymoseptoria tritici); spot blotch (Cochliobolus sativus) and tan spot (Pyrenophora tritici-repentis) [15, 17, 18]. The mechanisms by which Si protects plants against pathogens are mainly comprised of physical, biochemical, and molecular aspects, involving the strength of the cell wall and the formation of papillae, increasing the activity of defense-related enzymes, stimulating the production of antimicrobial compounds, activating the expression of defense-related genes, and regulating the hormone signaling pathways, such as salicylic acid, jasmonic acid, and ethylene [15, 17, 18].
Concerning the head blight-wheat pathosystem, available data are limited about enhancing adult wheat resistance against a disease that happened at the “head stage”. Under controlled conditions, granulated and foliar potassium silicate has a limited potential to decrease F. graminearum severity [19], however, disease reduction was observed on wheat and barley with the treatment of 1.50 g/kg of soil and 1.7 mM soluble silicon [20, 21]. Following field application, an increase in Si concentration in wheat tissues was associated with a reduction in Fusarium severity [22]. Nevertheless, there is no report that silicon application can enhance the resistance of wheat against FSB on young wheat parts in vitro. Thus, it is of value to investigate the utility of three distinct in vitro bioassays (LP of detached leaf inoculation, AUDPC of Petri-dish inoculation, and CLR of a coleoptile infection) to analyze the effect of Si in the wheat- FSB pathosystem.
The purpose of the current research was to assess levels of disease development of four Fusarium species causing FSB in wheat cultivars of contrasting susceptibility to disease grown with and without Si under in vitro conditions. Additionally, we wanted to explore whether application with Si could increase host resistance of susceptible to moderately susceptible cultivars measured by LP, AUDPC, and CLR to the same level of resistance exhibited by a moderately resistant wheat cultivar without Si.
2. MATERIALS AND METHODS
2.1. Plant Materials, Fungal Isolates, and Inoculum Preparation
Wheat cultivars Bohoth10 (bread, moderately resistant), Cham4 and Douma4 (bread, moderately susceptible), Cham7 and Cham9 (durum, susceptible to moderately susceptible), and Acsad65 (durum, susceptible) were chosen from previous in vitro, growth chamber, and field experiments to represent a range of quantitative resistance types to head blight [13, 14]. Isolates collected from diseased spikelets during the 2015 growing season originating from 9 different localities of Ghab Plain with a history of head blight epidemics, one of the principal Syrian wheat production areas, morphologically identified by the methods of Leslie and Summerell [23], and molecularly analyzed by random amplified polymorphic DNA [24] were selected for their contrasting pathogenicity (based on previous several experimental observations [13, 14, 24]. In total, 16 single-spore-derivedcultures of four Fusarium species, i.e., (F. culmorum (5 isolates), F. solani (6 isolates), F. verticillioides, synonym F. moniliforme (4 isolates) and F. equiseti (1 isolate)) were used in this study. Although F. graminearum is considered the major causative of head blight complex worldwide [2], this species was not found in the surveyed region (Ghab Plain) as observed in other studies investigating the composition of disease complex species in Ghab Plain during the spring of three seasons (2008-2010) [25]. Thus, the selection of pathogen species used in our study was reflective of other pathogen populations recovered from Ghab Plain and other principal Syrian wheat production areas [25, 26]; F. culmorum was the most frequent causing agent in Syria. Isolates were maintained in sterile distilled water at 4°C and frozen at -16°C until needed [27].
To produce conidia, Fusarium isolates causing head blight were inoculated into potato dextrose agar (PDA) with 13 mg/l kanamycin sulphate added after autoclaving, and shaken at 22ºC under continuous darkness for 10 days to allow mycelial growth and sporulation. Following incubation, cultures were covered with 10 ml of sterile distilled water, and spores were dislodged. Suspensions were filtered through two layers of sterile cheesecloth to remove the pieces of agar and mycelia and directly quantified under an optical microscope with a Neubauer chamber and diluted to desirable concentrations as inoculum sources.
2.2. Silicon Application
SiO2 powder (Kieselsaure, Carl Roth GmbH + Co. KG) with a minimum silicon content of 99% was used as the silicon source in this study. SiO2 powder was chosen as the preferred Si source, as there were indications that it reduced the incidence and severity of pathogenic FSB isolate on wheat and barley following artificial spike and spikelet inoculation in a growth chamber [20, 21]. A liquid solution of Si was prepared at the rate of 1.7 mM [28] since the application of this concentration reduced the bleaching of spikes and spikelets of wheat and barley plants under controlled conditions [20, 21].
2.3. Experimental Design
The utility of three distinct in vitro bioassays, i.e., latent period (LP) of detached leaf inoculation, area under disease progress curve (AUDPC) of Petri-dish inoculation, and coleoptile length reduction (CLR) of a coleoptile infection was investigated herein to analyze the effect of Si in the wheat-FSB pathosystem with modification in respect to Si application (Fig. 1). The experiments were laid out in a completely randomized design with three replications. The experiment was repeated three times.
Methods for LP assay were carried out as reported previously by Browne [9] to assess in vitro quantitative resistance components and utilized cited by Sakr [12] to assess resistance and pathogenicity in the wheat–head blight system. Seeds of wheat cultivars were disinfected with NaOCl for 8 minutes followed by 6 rinses in sterile distilled water (SDW). Then, wheat seeds were grown into plastic 15-cm pots including sterilized soil in a growth chamber with 20°C at day/night temperature and 16 h of light per day. The liquid formulation of silicon was first applied prior to planting, so the seeds were irrigated. Separate drenches were applied as 300 mL per pot once a week. SDW was used for controls. Seedlings were harvested after 14 days and 4 cm leaf segments were cut from the tip of the primary seedling leaf and then placed adaxial surface facing up on the surface of 0.5% water agar (four leaf segments per Petri-dish). Si- and SDW-leaf segments were inoculated at the center of the adaxial surface with a 10 μl conidialsuspension at 1×106 conidia/ml of the 16 fungal isolates. The uninjured detached leaves were then incubated at 10°C with a 12 h photoperiod. Assessments of symptom manifestation and sporulation were tested daily under a light microscope (magnification X40), and the resistance of a cultivar measured by LP was evaluated as a period of days from inoculation to sporulation. Three replicates of each cultivar based on observations on 120 detached leaves were set up.

Methods for AUDPC assay were conducted as described previously by Purahong et al. [10] to quantify in vitro aggressiveness components and cited recently by Sakr [13] to analyze both pathogenicity and resistance in the association of wheat and head blight fungi. Prior to infection, the 16 Fusarium cultures were covered with 10 ml of a Si-solution or SDW for controls. The mixture was filtered through autoclaved cheesecloth. The spore concentration was adjusted to 1×106 conidia/ml. Fifteen surface-sterilized wheat seeds were inoculated with 6 ml of a Si-suspension of each of 16 Fusarium isolates or SDW-suspension of each of 16 fungus isolates in the control treatment into a Petri-dish (9 cm in diameter) with sterile double-layer filter paper. The seeds were submerged under the fungal inoculum with/or without Si in the slanting Petri-dish then immediately aligned on the filter with the embryo turned upwards. To ensure high relative humidity and low air movement, Petri-dishes were then hermetically closed and sealed with 2 cm Parafilm strips. Infected and control treatments were incubated in an incubator at 22oC in the dark. The resistance of a cultivar measured by AUDPC was measured as the disease progressed over 6 days post inoculation (dpi) and its value ranged from 0 (very resistant) to 1 (not resistant) and calculated from the percentage of healthy coleoptiles as a function of time (from 2 to 6 dpi).
Methods for CLR assay were conducted as reported previously by Soresi et al. [11] to evaluate in vitro resistance components and cited recently by Sakr [12] to analyze both pathogenicity and resistance in the association of wheat and Fusarium fungi. Fungal suspensions with/or without Si were prepared as mentioned above with a spore concentration adjusted to 2 × 105 conidia/ml. Surface-disinfested wheat seeds in Petri dishes (10 seeds per Petri dish) were imbibed in 4 ml of a Si-suspension of each of 16 Fusarium isolates or SDW-suspension of each of 16 Fusarium isolates in the control treatment. After 15 minutes, the excess suspension was decanted and the inoculated wheat seeds were planted on filter paper placed on 0.5% agar in Petri dishes and were then incubated at 15ºC with a 16 h photoperiod. Coleoptiles were collected from each seedling and their lengths were determined using an analytical balance. Coleoptile measurements were recorded in each germinated individual and expressed as a percentage of the non-inoculated dish mean. The resistance of a cultivar measured by the CLR component was quantified at 6 dpi.
2.4. Statistical Analyses
Experimental data were subjected to analysis of variances (ANOVA) using DSAASTAT, 2015, version 1.514, Department of Agriculture and Environmental Science, University of Perugia, Italy. Arcsine transformation was used in the analysis of the percentage of coleoptile length reduction. The differences were compared using Fisher’s least significant difference test with a significant level of p<0.05. Single degree-of-freedom contrasts were used to make comparisons between specific cultivar groups, non-supplied and supplied with Si.
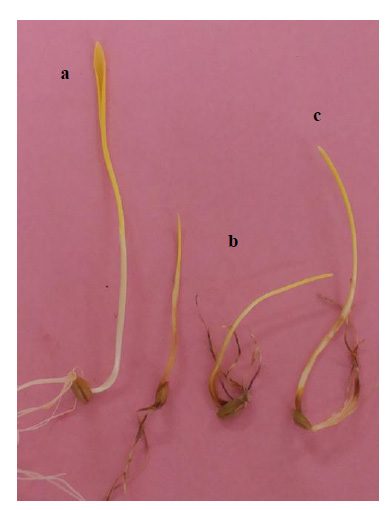
3. RESULTS
Differences were observed on wheat detached leaves and seedlings (Fig. 2) in the resistance of Si-Fusarium-inoculated treatments measured by LP (Fig. 3), AUDPC (Fig. 4), and CLR (Fig. 5) relative to fungal-inoculated-controls without Si, suggesting a strong effect of Si on reducing disease damage on the six tested wheat cultivars.
Application of Si concentration at 1.7 mM in detached leaves significantly (p<0.05) increased LP on the cultivar Bohoth10 which is moderately resistant; on the moderately susceptible cultivars Cham4 and Douma4, on the susceptible to moderately susceptible Cham7 and Cham9, and the susceptible cultivar Acsad65, by 18, 10, 13, 17% respectively in experiments 1; 20, 11, 14, and 16% in experiment 2, and 15, 12, 14, and 17% respectively in experiment 3, as compared with these cultivars without Si (Fig. 3).

AUDPC also decreased significantly (p<0.05) in seedlings of cultivars showed moderately resistant, moderately susceptible cultivars, moderately susceptible, and susceptible by 21, 16, 17, and 20% respectively in experiments 1; 18, 12, 16, and 18% in experiment 2, and 22, 17, 10, and 16% respectively in experiment 3, as compared with these cultivars without Si (Fig. 4).
Supply of Si in seedlings significantly (p<0.05) reduced CLR on Bohoth10; on Cham4 and Douma4, on Cham7 and Cham9, and Acsad65 by 18, 13, 11, 11% respectively in experiments 1; 14, 10, 12, and 15% in experiment 2, and 15, 11, 12, and 13% respectively in experiment 3, as compared with these cultivars without Si (Fig. 5).

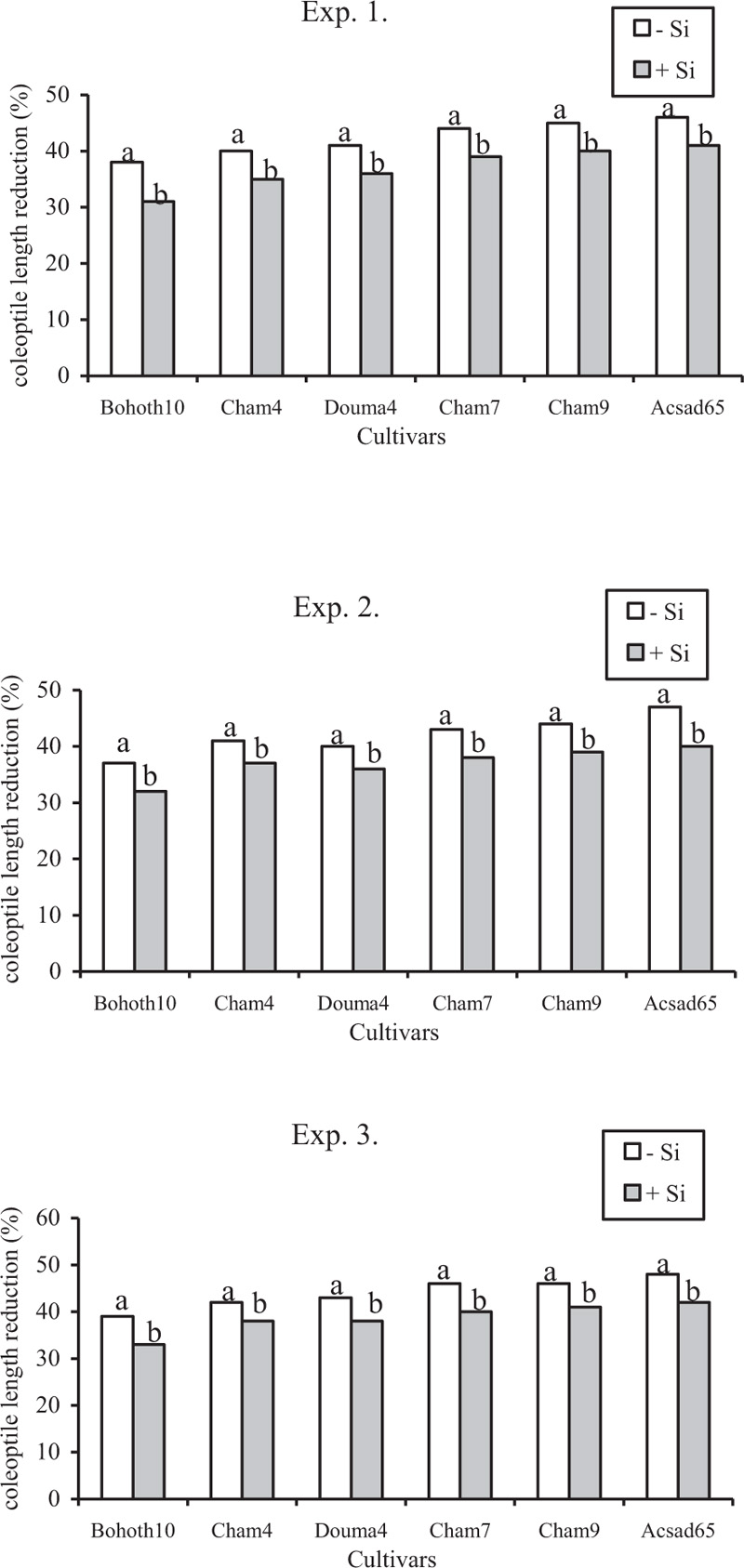
To determine whether the application of Si to moderately susceptible, susceptible to moderately susceptible, and susceptible cultivars could suppress the severity of head blight measured by LP, AUDPC, and CLR to a cultivar displayed a moderately resistant that had not been treated with Si, selected cultivar-Si combinations were compared with a single degree of freedom contrasts. No significant difference was detected for all comparisons at p<0.05. In all experiments, the combination of Cham4 and Douma4 and Cham7 and Cham9 or these cultivars analyzed separately including Acsad65, plus silicon reduced the severity of head blight measured by LP, AUDPC, and CLR to the same statistical level as that for the cultivar Bohoth10 which is moderately resistant without Si.
4. DISCUSSION
Si has been broadly used in enhancing resistance to a range of destructive fungal diseases [15, 17, 18], particularly in absorber and accumulator gramineous plants such as wheat [16]. Head blight is a worldwide problem in wheat; therefore, research has been extensively made to defeat it. Till now, few studies found that Si supply reduces Fusarium development in adult wheat plants in a growth chamber and field [19-22]. However, it is well known that such traditional resistance methods have limitations associated with time and space dependency [8]. The simple, rapid, and reliable in vitro evaluation of quantitative components in wheat against head blight pathogens indicate the potential of the detached leaf, Petri-dish, and coleoptile infection assays to (i) distinguish between specific sources/mechanisms of FSB resistance, and to (ii) combine multiple sources of disease resistance into future cultivars [12-14]. For the first time, we showed the utility of Si applied in three distinct in vitro bioassays (varying in inoculum concentration, infection methods, growth conditions, and target young plant parts) to reduce FSB development on young wheat parts.
Although quantitative wheat resistance to head blight is most often observed in field conditions, it may be (and often is) expressed in detached leaves and seedlings [9-11]. In our study, differences were observed in wheat seedlings and detached leaves in the resistance of Si-Fusarium-inoculated treatments relative to fungal-inoculated controls, showing the beneficial role played by this element in decreasing head blight disease symptoms on young plant parts under in vitro conditions. Appropriate in vitro conditions were determined for the detached leaf, Petri-dish, and coleoptile infection assays [12-14] to maximize differences in disease resistance components between treatments non-supplied and supplied with Si in six tested durum and bread wheat cultivars of contrasting FSB susceptibility. In parallel, previous reports showed that notable differences were found between treatments non-amended and amended with Si on young plant parts in some fungal-plant associations, i.e., pea seedlings infected with Mycosphaerella pinodes (leaf spot) [29], rice seedlings contacted with Pyricularia grisea (blast) [30], cotton seedlings infested with Fusarium oxysporum f. sp. vasinfectum (Fusarium wilt) [31], and potato detached leaves infected with Phytophthora infestans (late blight) [32].
The colonization pathway of Fusarium pathogens on a wheat spike at anthesis in plants supplied with Si has been well described [6, 7]. In the current research, we tried to solve the question of the extent and conditions in which in vitro Si application can defeat Fusarium pathogens by increasing wheat resistance measured by LP, AUDPC, and CLR. From an agricultural standpoint, answering this question demonstrated that the prophylactic effect of Si had manifested under the tested in vitro conditions, indicating that Si is absorbed in the form of silicic acid in young plant parts. Herein, we aimed to characterize the infection process of Fusarium pathogens on detached leaves and seedlings treated with Si at the early development stage.
Under favorable growth conditions in the Petri-dish and coleoptile infection assays [13, 14], wheat seeds absorbed fungal suspension for control or fungal suspension amended with Si to germinate with the appearance of the radicle. Then, as the first primary roots appeared; the coleoptile burst through the seed coat and began pushing towards the surface 5-6 dpi. For detached leaf inoculation [12], wheat seeds germinate after absorbing Si-solution or water for control. Then, the first leaf become visible above the soil surface at 14 dpi and seedlings were harvested to prepare detached leaves for fungal inoculation. According to Ma and Yamaji [33], specific Nod26-like intrinsic proteins as Lsi1 (Si influx transporter) facilitate the passive transport of Si across the plasma membrane from the environment (external solution) to the plant cell in the form of [Si(OH)4], and efflux transporters known as Lsi2 mediate the loading of Si into the xylem to facilitate root-to-shoot translocation, which, in turn, moves Si to the aerial parts where it is deposited as amorphous Si, (SiO2). Under our tested experimental conditions, roots quickly absorbed Si and then Si transferred to the aerial parts. In accordance with our findings, Lux et al. [34] observed the formation of silica aggregates in the root endodermis within 2 h following the transfer of a sorghum (absorber and accumulator gramineous plant as wheat) plant from a Si-poor environment to a Si-rich environment. Indeed, Si quickly precipitates as amorphous silica. Sangster et al. [35] studied the distribution of Si in a wheat plant within its growth. After only 8-10 days, Si was almost exclusively found as a solid form in the aerial parts.
Following contact with young wheat parts, Fusarium macroconidia initiated infection at the early development stage by forming an expansive hyphal network on the exterior surface of germinating seeds and detached leaves, where the epidermis is penetrated. At approximately 72 h post-infection, a dramatic increase in DON is observed. The increase in mycotoxins at this stage is associated with increased fungus proliferation and intracellular growth, which leads to early cell death [7]. Early production of DON may be associated with the suppression of plant defense mechanisms, representing the onset of pathogenicity-related gene expression in wheat and relatively higher gene expression overall for this host [36]. Taken into account that Si did not act directly on the pathogen [37], the effect of Si on the reduction of FSB intensity under in vitro conditions in detached leaves and seedlings can be attributed to two mechanisms that act additively and/or synergistically: physical barrier and biochemical defenses [15, 17, 18]. First, the physical barrier may be to be due to Si deposition below the cuticle and the increase of papillae deposition at infection sites. Second, Si may prime the biochemical defenses by increasing defense enzyme activity, biosynthesis of phenolic compounds and phytoalexins, and accumulation of hydrogen peroxide at the infection sites. Further studies are required to explore whether Fusarium species show any difference in infecting durum wheat or bread wheat cultivars.
Quantitative resistant wheat cultivars are identified by long LP and less AUDPC and CLR determined in vitro [12-14]. In all bread and durum wheat cultivars of contrasting susceptibility to disease infected with different Fusarium species, the application of Si did increase host resistance measured in vitro; 1.7 mM Si resulted in significantly higher LP and lesser AUDPC and CLR compared with controls without Si. Since Si is known to reduce the intensity of fungal diseases in different crops [15, 17, 18], the level of this element in the young wheat parts was carefully equilibrated in all treatments to express its potential ability in the suppression of FSB. In field studies to determine the efficacy of Si for the prevention of plant diseases, Si concentration does not exceed 1.67 mM [38]. Such Si concentration was fulfilled under growth chamber conditions to enhance Type I and Type II in the spikes of wheat and barley to head blight [20, 21]. This observation suggests that in wheat supplied with Si, the development of the FSB pathogens was slowed down, and might be due to the two mechanisms mentioned above.
Since Si did not supply in detached leave- and seedling- controls, it can be concluded that variations in Si accounted for differences in the level of disease response obtained herein. Our data have been substantiated by Rodrigues et al. [39] through their study on sheath blight, caused by Rhizoctonia solani. Rice cultivars grown at the highest Si rate had sheath blight intensities that were greatly reduced in comparison with cultivars grown in pots not amended with Si [39]. Increasing Si concentration in detached leaves and seedlings significantly reduced the severity of FSB measured by LP, AUDPC, and CLR on the cultivar Bohoth10 that is moderately resistant; on the moderately susceptible cultivars Cham4 and Douma4, on the susceptible to moderately susceptible Cham7 and Cham9, and the susceptible cultivar Acsad65. More importantly, Si at 1.7 mM augmented host resistance of susceptible to moderately susceptible cultivars measured by LP, AUDPC, and CLR to the same level of resistance exhibited by a moderately resistant wheat cultivar without Si. In accordance with our findings, Rodrigues et al. [40] found that Si significantly reduced the severity of sheath blight, i.e., moderately susceptible US rice cultivars treated with Si were as resistant as a partially resistant cultivars without Si.
CONCLUSION
This is the first report presenting the utility of three distinct in vitro bioassaysto investigate the effect of Si in the wheat-FSB pathosystem. More importantly, wheat cultivars grown at the concentration of 1.7 mM had FSB intensities that were greatly reduced assessed by higher LP and lesser AUDPC and CLR in comparison with cultivars not amended with Si. Irrespective of pathogenic and botanical origin for the fungal and plant materials in our study, Si enhances wheat resistance to Fusarium infection, suggesting that Si exerts no selective pressure on pathogen populations, and therefore it can protect any durum and wheat cultivar whatever its quantitative resistance from infection with any Fusarium species whatever its pathogenic level. Findings generated in this research, even though the experiments were conducted under in vitro conditions, propose that silicon has the potential to complement inherent host resistance and decrease head blight intensity. Among the wheat cultivars tested, Bohoth10 showed a moderate resistance to head blight, especially in combination with Si. The application of silicon to complement host resistance to head blight appears to be an effective strategy for disease management in wheat.
LIST OF ABBREVIATIONS
| Si | = silicon |
| FSB | = Fusarium Seedling Blight |
| LP | = Latent Period |
| CLR | = Coleoptile Length Reduction |
| AUDPC | = Area Under Disease Progress Curve |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The author would like to thank the Atomic Energy Commission of Syria for financial support.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank the Atomic Energy Commission of Syria for assisting with this research. The unknown three Reviewers are also thanked for their constructive comments on this manuscript.


