All published articles of this journal are available on ScienceDirect.
Evaluation of the Effects of New Environmental Additives Compared to Mineral Fertilizers on the Leaching Characteristics of Some Anions and Cations under Greenhouse Plant Growth of Saline-Sodic Soils
Abstract
Aims:
The aim of this study was to determine and monitor the influences of organic and biological additives compared to mineral fertilizers on leaching characteristics of anions and cations, also to know more about the ability of these additives to make complexes with dissolved and toxic salts to decrease soil salinity.
Background:
Salt-affected soils comprise of saline and sodic soils which differ in origin, physico-chemical properties and the constraints to plant growth. Due to the presence of excess soluble salts (e.g. sodium (Na+) and chlorides (Cl-)).
Methods:
Ten treatments were established, including two levels of spent grain (environmental organic wastes from the beer industry), S1(10 g of spent grain / kg soil) and S2 ( 20 g of spent grain / kg soil); two levels of compost M1(10 g of compost / kg soil) and M2 ( 20 g of compost / kg soil); mixed M1 with S1 (M1S1); inoculation of Azospirillum brasilense (A1); inoculation A1 with S2 (A1S1); inoculation A1 with M1 (A1M1); 20:20:20 of N, P, and K fertilizers (NPK), and control (CK, without any additives). All treatments were mixed with 30 kg soil pots under greenhouse conditions, corn (Zea mayz L.) seeds were sown in the soil pots. The most relevant nitrogen and salt in soil leachates were collected and analysed every 20 days for 100 days. The soil leachates were collected under plant growth from pots by closed system.
Results:
The result revealed that organic additives such as A1 and S2 treatments effectively decreased soil pH, soil EC, and reduced NaCl concentration in soil leachates. The Ca2+ and K+ cations in the soil leachates were not stable at high levels of organic additives. Soluble NO3- and NH4+ were significantly lower in A1, S2, and A1S1 treatments than in NPK, M2, and CK treatments. Soil treatment with A1 and S2 significantly improved the soil chemical environment by increasing the Cation Exchange Capacity (CEC) and soluble and exchangeable-K+ and thus limited entry of Na+ into the exchange complex in soil and consists complex with soluble Na+ as sodium humate form.
Conclusion:
In the final, the highest nitrogen use efficiency with the least NO3- and NH4+ losses in saline-sodic soil was also found in S2 and A1 treatments. Moreover, under this bio-organic fertilization way, NO3- concentrations in soil leachates was outside of danger of damaging the environment. Thus, spent grain with Azospirillum were suggested to be the optimal fertilizer with the lowest nitrogen leaching losses, best yield, quality, and the least groundwater environmental risk under corn (Zea mays L.) organic and bio-organic cultivation comparing with chemical cultivation.
1. INTRODUCTION
The ever increasing world population is worrisome in relation to the fulfillment of global food requirements that may double by 2050. Over the same period, natural resources are predicted as limiting because of rapid global climate change. Owing to the use of brackish irrigation water, water-logging, and natural subsoil salinity, fertile soils are becoming salt-affected and a major threat to sustainable food production in arid and semiarid areas of the world [1]. The term salinity refers to the accumulation of the specific amount of soluble salts (electrical conductivity (EC) ≥ 4 dS m–1) in the root zone, which inhibit plant growth. Soils in which the sodium adsorption ratio (SAR) concentration is increased (SAR ≥ 13) at the exchange sites of the soil particles but with a low concentration of total soluble salts (EC< 4 dS m–1) are described as sodic soils. Those soils with high soluble salt concentrations EC ≥ 4 dS m–1and SAR ≥ 13 are termed saline-sodic soils [1].
Incorporation of Spent Grain (SG) is the organic waste from the beer industry. It is acidic and rich in organic matter and nutrients. In Egypt, spent grain has no value and is available at no cost all year. SG application in highly saline-sodic and alkaline soils improved the physico-chemical and biological properties as evident from significant reductions in soil pH and exchangeable sodium, Na+ chelation, increase in soil carbon and nitrogen and enhanced activity of urease enzyme [2]. Organic matter addition increased the soil structural stability, improved the filtration rate and enhanced the microbial biomass. Amended soils showed lower water-soluble of Na+ and better biochemical properties as compared to control plots [3].
Application of Plant Growth Rhizobacteria (PGPR), such as Azospirillum brasilense as a bio-fertilizers bacteria enhanced salt tolerance of salinized (4 g l −1 NaCl) Zea maize L. Azospirillum inoculation led to decrease the concentrations of Na+, Cl- and NO3--N in soil leachates and thus alleviated solubility of salt effect on biomass production [2, 4]. Soil organic and biological significantly improved the soil chemical environment by increasing the Cation Exchange Capacity (CEC) and soluble and exchangeable-K+ contents and thus limited entry of Na+ into the exchange complex in soil by sodium-humate. The concentrations of K+ and Ca2+ in soil leachates by these amendments also accounted for better crop nutrition and growth [5]. Application of organic amendments, such as compost and biochar, increased soil respiration rate and soil microbial bio-mass in saline soil, and significantly improved the cumulative SOC and total nitrogen in comparison to both gypsum application and control treatments [6, 7].
Organic wastes amendment positively affect the chemical and physical properties of the soil which include pH that led to direct absorption of NH4+–N and NO3--N, great Cation Exchange Capacity (CEC) and improved Water Holding Capacity (WHC) of the soil, reducing the volume of leachate [8, 9]. Many early studies stated that effective soil management practices reduce nitrogen contamination, especially NH4+-N and NO3--N and reduce pollution of agriculture wastewater
[10, 11]. It has been reported that bio and organic fertilizers affected positively on reducing NO3—N levels in soil leachates; however, there was an offset by the decrease in N mineralization and N soil fixation by microorganisms [12].
A new study has focused on a positive effect of SG and Azospirillum on NO3—N, Cl-, and Na+ levels in soil leachates and NH4+ losses in calcareous soils [2, 13]. However, few studies on the role of SG and Azospirillum compering with mineral applications under the greenhouse were found [14, 15]. It is interesting to conduct a study to enhance nutrients availability and to reduce NO3--N leaching by using the application of spent grain with Azospirillum in soil under maize plant growth. The combined bio-spent grain additives with mineral fertilizers are considered to be necessary to promote plant productivity, decrease environmental risk, and protect nitrogen levels in the soil by lower NO3-N leaching in groundwater.
The aim of this study was to determine and monitor the influences of organic and biological additives compared to mineral fertilizers on the Na+, Cl-, NO3-, and other nutrients concentrations in soil leachates; also discover more about the ability of these additives to make complexes with dissolved and toxic salts to decreasing soil salinity.
2. MATERIALS AND METHODS
2.1. Study Area and Soil Characterization
The study area has a continental climate condition with hot summer and wet winter. The lowest temperature values were pronounced in June and September (32○C and 25○C), whereas the maximum rainfall amount is approximately 14.7 mm/month in February [15]. The soil in the study has a clay loam texture and can be classified as calcareous saline-sodic soil. The samples of the air-dried soil were used for the physical and chemical parameters of the saline-sodic soil, as shown in Table 1.
2.2. Soil Additives and Experimental Design
Three types of treatments were used, the first one Azospirillum brasilense as the bio-fertilizer bacteria. The second was compost consisted of plant and animal wastes. The last one was spent grain, a by-product of the beer industry. The main characteristics of the organic wastes were determined according to the standard procedures, as shown in Table 2. Ten treatments were established, including two levels of spent grain, S2 (20 g kg-1 soil) and S1 (10 g kg-1 soil); two levels of compost, M2 (20 g kg-1 soil) and M1 (10 g kg-1 soil); a mix between M1 with S1 (M1S1); inoculation of Azospirillium, with seed and soil (A1); A1 + S1, (A1S1); A1 + M1 (A1M1); mineral fertilizers 20: 20: 20 of N, P, K (NPK), and control (CK, without fertilizers). All treatments were mixed with 30 kg soil pots under greenhouse conditions for the 120 days of corn seed sowing. The most relevant nitrogen leaching forms were collected and analysed every 20 days for the 100 days. The soil drainage was collected under the pots by a closed system.
| Parameter | Saline-sodic Soil |
|---|---|
| pH (1:2.5 w: w) | 8.84 ± 0.05 |
| ECe (dS m-1) | 5.43 ± 0.10 |
| Total N (gkg-1) | 0.02 ± 0.001 |
| Available P (mgkg-1) | 1.20 ± 0.07 |
| Available K+ (mgkg-1) | 78.1 ±0.44 |
| Total CaCO3 (%) | 18.6 ± 1.35 |
| CEC (cmol+ kg-1) | 7.56 ± 0.24 |
| Organic Matter (g kg-1) | 1.78 ± 0.21 |
| Soil Organic Carbon (g kg-1) | 1.03 ± 0.01 |
| Total DOC* (%) | 0.012 ± 0.003 |
| ESP** (%) | 53.1 ± 1.93 |
| С:N Ratio | 49.14 ± 2.1 |
| Sand (%) | 31.2 ± 0.11 |
| Silt (%) | 23.5 ± 0.21 |
| Clay (%) | 45.3 ± 0.18 |
| Texture | Clay Loam |
| Micronutrients DTPA Extractible (mgkg-1) | |
| Fe2+ | 0.08 ± 0.01 |
| Zn2+ | Nd* |
| Mn2+ | 0.11 ± 0.020 |
| Cu2+ | 0.003 ± 0.01 |
| B+ | Nd* |
| Cl- | 192 ± 0.166 |
| Parameter | Compost | Spent Grain |
|---|---|---|
| pH (1:5 w:w) | 7.20 ± 0.01 | 4.16 ± 0.03 |
| EC (dS/m, 1:5 w:w) | 5.81 ± 0.21 | 1.45 ± 0.21 |
| Organic Matter (g kg-1) | 332 ±1.23 | 750 ± 0.57 |
| Total N (%) | 2.10 ± 0.32 | 3.12 ± 0.68 |
| Total P (%) | 1.03 ± 0.52 | 1.86 ± 0.54 |
| Total K (%) | 0.57 ± 0.01 | 1.74 ± 0.63 |
| C: N ratio | 9.16 ± 0.35 | 13.9 ± 0.12 |
| Organic carbon (%) | 19.25 ± 0.12 | 43.5 ± 0.94 |
| Fe2+ (mgkg-1) | 960 ± 2.97 | 1130 ± 3.87 |
| Zn2+ (mgkg-1) | 220 ± 5.34 | 368 ± 2.34 |
| Mn2+ (mgkg-1) | 100 ± 2.14 | 210 ± 1.98 |
| Cu+ (mgkg-1) | 61 ± 1.21 | 98 ± 1.54 |
The experiment was conducted in a greenhouse during the corn (Zea Mays L.) growing season. Ten treatments with three replicates were carried out, namely S2, M2, S1, M1, A1, A1S1, A1M1, S1M1, NPK, and CK. The experimental area then consisted of 30 pots, each post 30 kg soil, and these 30 pots were arranged as split plots in a randomized complete block with a 30 cm isolation strip in order to avoid interference. The control treatment was without fertilization. Maize crop has a high water demand under arid condition. The irrigation period starts in June and ends in early September. The number of irrigation events depends on the irrigation method, soil type and the cropping system. The mean water amount which applied per pots cropping season from June to Sept was 250 mm from June to July, and 500 mm until the end of the experiment.
2.2.1. Azospirillum as Bio-fertilizer
Azospirillum brasilense (Sp245) was cultured; growth and inoculation with seed and soil were performed as described by [16]. Briefly, the Azospirillum bacterium was grown for 4 days in LB medium (10g L-1 triptone, 5g L-1 NaCl, 10g L-1 yeast extract) with continuous shaking at 30°C. Bacteria were then centrifuged for 15 min at 4500 x g and washed four times with sterile saline solution (NaCl 0.85% w/v); after that, the Azospirillum bacteria became ready for inoculation with the soil.
2.3. Leachates Sampling and Analysis
The nitrogen loss through water leaching was collected at 20 days intervals started at three weeks after transplanting until three weeks before harvesting. The samples were collected at the leaching pipe of the drainage system then store at 4 Co before analysis and measured using a 50 mL graduated cylinder. The NO3--N concentration was analyzed using the cadmium reduction method on a spectrophotometer at a wavelength (500 nm). The NH4+-N concentration was analyzed using the Nessler method [17]. The intensity of the color is determined by a compatible photometer and the concentration based upon the meter, which will be presented in mg L– 1 (ppm) of NO3– –N and NH4+–N in l leachates. The total NO3– –N and NH4+ –N leaching loss was calculated by multiplying the N concentration to the leachate volume.
In order to calculate the actual nitrate loss through soil pots, we considered the nitrogen concentration of the soil solution at the bottom depth of the explored soil pots and the water draining through it. Nitrate leaching was estimated using the trapezoidal rule using a Multi-parameter photometer with COD (HI83399) instrument as proposed [18], which assumes that nitrogen concentrations in the extracted soil water solution represented mean flux concentrations. The total nitrogen leached over each sampling interval, in mg kg-1, was calculated as N leached = 0.5 (C1 + C2) V / 100 where C1 and C2 are successive pairs of sampling occasions (mg NO3-–N L−1), and V is the drainage volume between sampling occasions (mm).
2.4. Soil and Organic Wastes Analysis
Total thirty samples referred to ten treatments × three replications; soil samples were taken from each bottle after the removal of visible roots and fresh litter material, the composite samples were sieved (2 mm) and then stored at room temperature for less than three months until chemical analyses were performed. Samples were air-dried and ready for measured. The Particles-size distribution was determined by the hydrometer, as described [19]. Basic characteristics, such as pH of the soil was measured potentiometrically in a soil: water suspension 1:2.5 w/v [19]. The electrical conductivity (EC) was measured in saturated paste extracts using an EC meter [20]. The soluble calcium (Ca2+) and chloride (Cl-) were extracted with soil-water 1:5 w/v and measured by titration method [18]. Soluble sodium (Na+) and potassium (K+) were extracted with soil-water 1:5 w/v and determined by flame photometer [17]. The total nitrogen (N) was determined by the Microkjeldehl method [17], available phosphorus (P) was extracted by blue methods with 0.5 N NaHCO3 [21]. The available potassium (K+) was extracted by 1 N ammonium acetate solution and measured by the flame photometer. Soil organic carbon (SOC) concentration was determined by oxidization with K2Cr2O7 [21].
2.5. Statistical Analysis
Statistical analysis was carried out using the SPSS v.16 software (Visauta, 2007). Data were submitted to a normality test before the analysis of variance. When statistical significance was found (P ≤ 0.05), a comparison of the means was carried out using the Tukey test. Furthermore, a Pearson correlation analysis was carried out to observe the degree of association between some of the studied variables.
3. RESULTS AND DISCUSSION
3.1. Leachate Properties of Treated Soil
The effects of organic and bio-organic ameliorants on the properties of successive leachates from the saline-sodic soil. The first leaching was conducted on soil, which had been slowly wetted to 85% of field capacity; subsequent leaching was conducted on air-dried soil. After five-time leaching every 20 days, there were significant changes in leachate properties imposed by spent grain and Azospirillum treatments for soil. As from the 20 to 100 days was analyzed for the EC, pH, NH4+, NO3-, Na+, Cl-, Ca2+, and K+ data for these leachates are presented in the following section.
3.1.1. Effects of Organic and Bio-Organic Additions on the pH Values in Soil Leachates
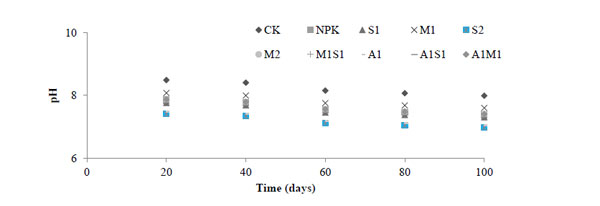
Fig. (1) show the pH values of the saline-sodic soil treated with organic and bio-organic ameliorants. The soil leachates at S2 alone decreased pH, but these effects were reversed in the NPK and CK treatments. In the A1 and S2 treatments, the decrease of pH was enhanced by the addition of both treatments. However, NPK and M2 treatments increased pH in the soil, while A1M1 did the opposite. The lowest pH was observed in the S2 treatment, and the highest ph values were observed in the CK and NPK treatments in saline-sodic soil after 100 days of seed sowing. The increase in pH of soil by the addition of M2 and M1 was most likely due to CO3-2 and Ca2+ produced when compost was applied to the soil. However, the increase in pH of soil may induce the Ca2+ to become more alkaline and, therefore, more sodic as the solubility of Ca2+ is suppressed [2]. In arid and semi-arid regions, the soluble Ca2+ and Mg2+ become low, and Na+ and K+ ions accumulate in soil solution when the concentrations of CO32 and HCO3- increase [7, 22, 23]. Organic acids and CO2 produced during incubation of spent grain and Azospirillum might reduce pH in the soil. However, the magnitude of this decrease brought about by 10 t/ha of spent grain did not lower the pH of the soil sufficiently to influence lime solubility. Further experiments are needed to determine the best combination rates of spent grain and Azospirillum to overcome problems associated with sodicity. In the saline-sodic soil, the pH was increased when compost and NPK were added. This result was observed in Fig. (1).
3.1.2. Effects of Organic and Bio-Organic Additions on the EC Concentrations in Soil Leachates
Fig. (2) show the concentrations of the electrical conductivity (EC) for 100 days in the saline-sodic soil leachate followed the order NPK > M1 > M2 > CK > M1S1 > A1M1 > S1 > S2 > A1 > A1S1. Additionally, A1S1 lost the salt concentrations in soil leachates more than the control and NPK treatments, and S2 lost more than M1S1 did. The EC concentrations of leachates decreased from 20 to 100 days of seed sowing in virtually all treatments. The EC was greatest in the initial leaching (20 days) and lowest in the last leachates (100 days) in all treatments, including the control. This indicates that cation dissolution was greater in the early stages and diminished as successive leaching proceeded. In bio-fertilizer treatments, the trend of EC concentrations was to decrease as leaching proceeded was similar to the spent grain with Azospirillum treatments. The EC of each leachate in the NPK and M2 treatments were almost invariably significantly higher than in the leachates of all other treatments at the same leaching events. Minor but consistent reduction in the EC occurred for all leaching events [7, 22, 24]. The EC of soils was increased by adding NPK and compost fertilizers because both treatments provided an increase in electrolyte concentration. When A1+M1 was applied to the soil, the increase in EC was not significantly higher than that of M1- only. This indicates that the application rates of compost (10 and 20 g kg-1 soil) may be too low to affect the solubility of salts and that either the rates may need to be increased to enhance the solubility of lime or that more time is required to observe the desired effect. Further experiments are needed to answer this question [7, 22].
3.1.3. The NO3- and NH4+ Concentrations in Soil Leachates
Fig. (3) shows the trends of nitrate leaching from 20 to 100 days of seed sowing. Nitrate (NO3-) leaching losses in each treatment decreased as the spent grain increased. Fig. (1) shows the concentrations of the NO3- leaching from 20 to 100 days in the saline-sodic soil followed the order NPK > M2 > A1M1 > M1 > M1S1 > A1S1 > S2 > S1 > CK > A1. Additionally, A1 lost more than the compost and NPK treatments, and S2 lost more than A1M1 did. Nitrate leaching losses during the corn experiments from 20 to 60 days were lower than the losses in the period from 60 to 100 days. These results indicated that spent grain and Azospirillum application were beneficial for reducing nitrate leaching.

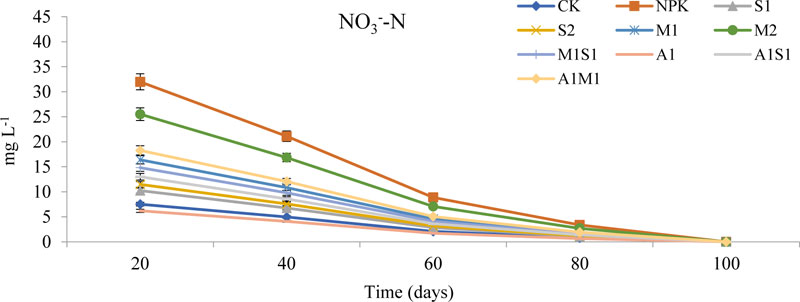
S2 and A1 treatments reduced the NO 3 - leaching losses in the soil layers. The treatment NPK and CK treatments significantly differed (P < 0.05), the exception that no obvious differences between CK and S1 from 20 to 100 days (P > 0.05) were observed. The nitrate leaching losses from 20 to 40 days ranged from 31.98 to 14.82 mg L-1 in the NPK and S2, respectively, and in 40 to 60 days leaching losses from 21.10–8.56 mg L-1 in the NPK and S2, respectively. No significant differences were observed between the compost and NPK treatments. Below the NO3- concentration, no significant differences (P > 0.05) were observed among A1, S1, and CK. The results for the 100 days show that S1 and A1 applications could reduce soil nitrate leaching losses in the soil layer. The losses in both S1 (11.46 mg L-1) and A1 (4.08 mg L-1) was significantly differed compared with NPK (31.98 mg L-1) (P < 0.05) after 20 days of seed sowing. The presented results of NO3- are in agreement with the results [8].
On the other hand, the organic additives nitrate concentration is the key factor influencing Ammonium (NH4+) leaching losses. Fig. (4) shows the course of changes in the soil NH4+ concentration in the soil layers at different time points. In the corn pots, the annual mean of the NH4+ concentrations were higher (P < 0.05) from 20 to 100 days. The trends for NH4+ were very similar to trend NO3-. The most critical period of leaching was during 20 days because this start time for amendments of organic wastes to soil, and the NH4+ concentration was much higher during 20 days than other periods from 40 to 100 days of measurements (P < 0.05). After 40 days of seed sowing the NH4+ concentration in water, leaching was zero from 40 to 100 days, except M2 treatment was 4 mg L-1.
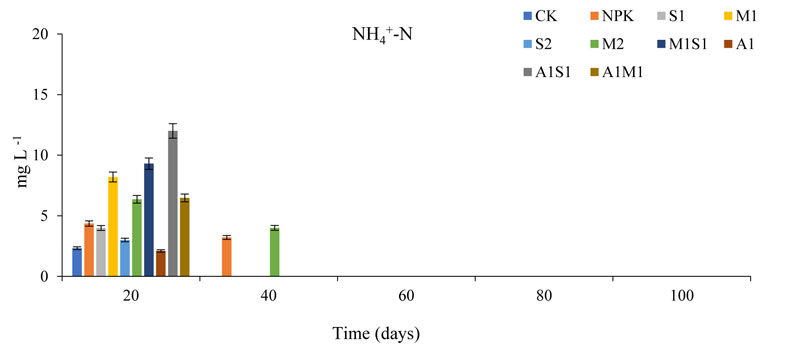
Not surprisingly, the period of start for organic additive decomposition was higher for NH4+ leaching. The NH4+ concentration after 20 days in A1 treatments was lower than that in CK and NPK treatments compared with M2 and A1S1 treatments, the NH4+concentration in the S2 and A1 treatments both significantly differed (P < 0.05) after 20 days; no significant differences were observed between all treatments in Aug or Sept in NH4+ leaching. However, significant differences were observed between the CK and S2 treatments at all-time points (P > 0.05).
Organic applications increase the capacity of soil to retain N as both NO3--N and NH4+-N. However, biochemical processes are involved in controlling NO3- leaching through the soil column. Therefore, it is difficult to anticipate NO3- retention by spent grain and biological additives in the soil system. In addition, spent grain with Azospirillum inoculation has a substantial capacity to adsorb NO3- and NH4+ from the soil solution. It can reduce the rate of N mineralization and NO3--N leaching from the soil layers due to adsorption of NH4+ and organic nitrogen produced during mineralization of soil organic matter [9, 25]. Also, NO3--N may be lost via other pathways, such as denitrification and immobilization [26].
The results show that spent grain and Azospirillum applications were beneficial for reducing the soil nitrate and ammonium concentrations during the early stages of corn growth. However, in the middle and at the end of the corn growth stage, the soil NH4+ leaching did not increase despite the soil nitrogen concentration increasing slightly. Although the bio-spent grain applications constituted an additional source of nitrogen, the NO3- leaching losses did not increase. The presented results of NH4+ and NO3- are in agreement with the results [8, 18].
3.1.4. Effects of Organic and Bio-organic Additions on the Na+ Concentrations in Soil Leachates
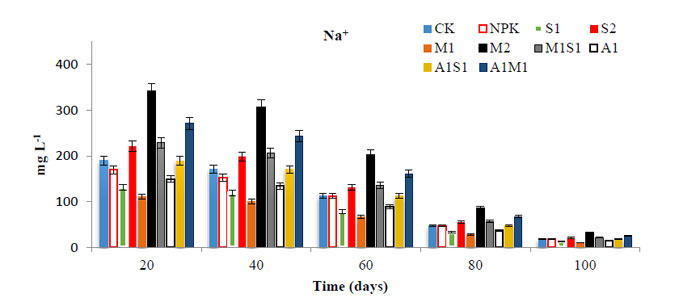
The sodium (Na+) concentrations in the soil leachates are shown in Fig. (5). The concentrations of Na+ leaching from 20 to100 days in the saline-sodic soil to ground-water followed the order M2 > A1M1 > M1S1 > M1>A1S1 ≥ CK > NPK > A1 >S1> S2. According to the reported Na+ concentrations, S2 and A1 application rates were lowest compared to the M2 application rates. The organically treated soil with a mixture of M2, A1M1, and NPK possessed the highest Na+ concentrations among the treatments. The trend amount of Na+ and Cl- concentrations decreased with increasing leaching period from 20 to 100 days, (Fig. 6). As the application level of compost increased, the NaCl concentration increased. There were significant differences among the treatments in the Na+ and Cl- concentrations. Soil treatment with A1 and S2 significantly improved the soil chemical environment by increasing the Cation Exchange Capacity (CEC) and soluble-exchangeable-K+ contents and thus limited entry of Na+ into the exchange complex in soil and consists complex with soluble Na+ as sodium humate forms [27]. Although the Azospirillum application constituted an additional source of salts, the Na+ and Cl- leaching losses did not increase [2]. High sodium chloride can lead to deterioration of soil physical properties and increase water salinity and toxicity. Soluble sodium and chloride can adversely affect soil structure by making the soil very susceptible to crusting, impeding water infiltration, and hindering root growth [28].
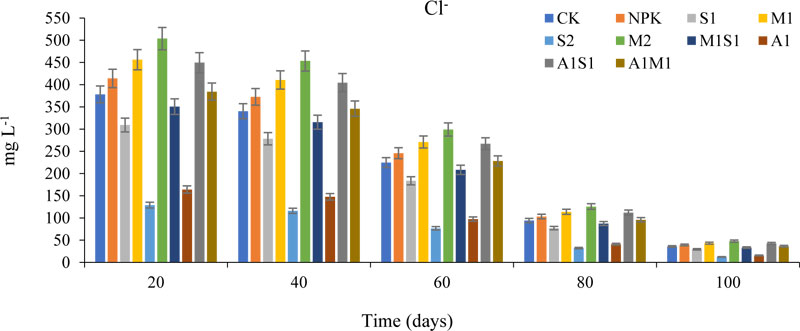
3.1.5. Effects of Organic and Bio-organic Additions on the Cl- Concentrations in Soil Leachates
The Chloride (Cl-) concentrations after 20 days of seed sowing were 503.62, 456.10, 449.66, 413.88, 384.34, 378.09, 350.64, 309.24, 164.01, and 128.93 (mg L-1) for the M2, M1, A1S1, NPK, A1M1, CK, M1S1, S1, A1, and S2 respectively. Fig. (6) shows the concentrations of Cl- leachate from 20 to 100 days in the saline-sodic soil followed the order M2 > M1 > A1S1 > NPK > A1M1 > CK > M1S1 > S1 > A1 >S2. According to the reported concentrations of Cl-, in S2 with A1, treatments were lowest compared to compost and NPK application rates. There were significant differences among the means of the studied treatments. The spent grain and Azospirillum did not increase the Cl- concentration relative to the other treatments. Therefore, the spent grain and Azospirillum could not lead to a salinity hazard and water toxicity by chloride. The M2 treatment possessed a high concentration of Cl- concentrations compared to control and NPK treatments after 100 days of soil leachates. The compost source increased the chloride concentration in saline-sodic soil significantly compared to S2 and A1. The differences in the concentrations of Cl- were significant among the treatments used. The superiority of the spent grain was due to initially high organic matter, low pH, and high water holding capacity. The high concentration of Cl- provided by the M2 raises the risk of salinity hazard for soils. Therefore, the M2 applications used in the present study are not favorable in soil fertilization or reclamation compared to the S2 and A1. The increased concentration of Cl-ions in any organic source increases osmotic pressure and decreases water potential, making it harder for plants to take up water. Additionally, high concentrations of Cl- could cause toxicity to some plants [2, 28]. When using an organic source, it is important to know the effect of source quality on the salt content of the soil solution. All common chlorides are soluble and contribute to the total salt content of soils. However, chloride is an essential element for plant nutrition but it is needed in a small quantity.
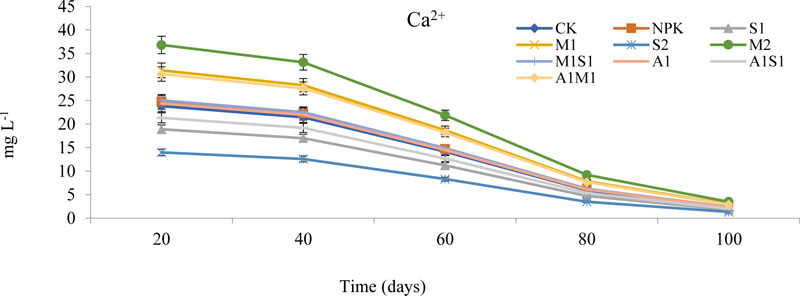
3.1.6. Effects of Organic and Bio-Organic Additions on Ca2+ and K+ Concentrations in Soil Leachates
The calcium (Ca2+) and Potassium (K+) concentrations in soil varied considerably during the study period are shown in Fig. (7) and (8). The concentrations of the Ca2+ leaching from 20 to 100 days in the saline-sodic soil. The Ca2+ concentration was lower in the bio-spent grain treatments than in the compost and NPK treatments. The Ca2+ leaching concentrations in water leaching were followed the order M2 > M1> A1M1 >M1S1 > A1 > NPK > CK > A1S1> S1 > S2, respectively. The S2 treatment positively affected Ca2+ concentration compared to other organic treatments. The S2 treatment supplied the lowest calcium concentration while the M2 supplied the highest in soil. Ca2+ efficiency due to higher compost of Ca2+ is rare but may occur on alkaline and saline soils. The Ca2+ is generally not deficient in soils when pH is 7.5 or above. Therefore, the soil used in the present study has sufficient Ca2+ needed by a growing plant and decreasing Na+ adsorption concentration. On the other hand, the concentration of K+ was similar to the trend of Ca+2 content presented earlier. The organically treated soil with a mixture of M2, A1M1, and NPK possessed the highest K+ concentration among the treatments after 20 days. The application level of compost increased K+ concentrations.
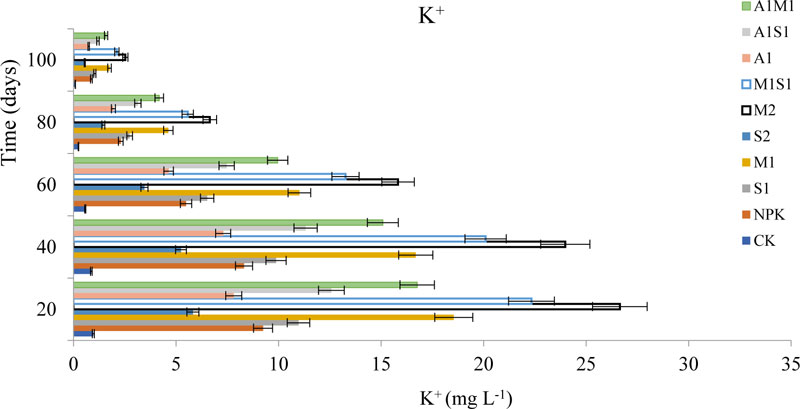
The variation in K+ in saline-sodic soil may be due to the activity of bacteria in the soil affecting the decomposition process. However, adding organic materials can significantly increase and maintain K+ in S2 and A1 treatments more than in the CK treatment. Plants grown under saline-sodic conditions take up low amounts of K+ by the roots because of cationic competition between K+ and Na+, which can cause K+ deficiency. The S2 treatment significantly increased (p < 0.05) the CEC of both systems, possibly due to increased surface area after pyrolysis of spent grain and charge density. The results show that spent grain and Azospirillum applications were beneficial for reducing the soil calcium and potassium concentrations during the early stages of corn growth from 20 to 100 days [14]. The application of spent grain with Azospirillum significantly increased soil fertility and plant growth in this results [13, 29, 30].
CONCLUSION
Spent grain with biological additives significantly decreased concentrations of the EC, Na+, and Cl- in soil leachates compared with compost and NPK treatments from 20 to 100 days, but NPK fertilizers and compost organic wastes applications may cause serious environmental risk. The high concentration of Cl- provided by the M2 raises the risk of salinity hazard in soils. The highest nitrogen use efficiency with the least NO3- and NH4+ losses in saline-sodic soil was also found in S2 and A1 treatments. Moreover, under this bio-organic fertilization way, NO3- concentrations in soil leachates was outside of danger of damaging the environment. Thus, spent grain with Azospirillum as a bio-organic additive was suggested to be being the optimal fertilizer application with the lowest nitrogen losses in water, grain yield for corn plants, and highest soil fertility, compared with chemical cultivation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the Colleagues of Department of Soil Science and Soil Ecology, Institute of Earth Sciences of St. Petersburg State University, Saint Petersburg, Russia, Colleagues of Department of Land and Water Technologies, Arid Lands Cultivation Research Institute, City of Scientific Research and Technological Applications, Alexandria, Egypt, and Cultural Affairs and Missions Sector, Ministry of Higher Education and Scientific Research, Egypt and Russia.


