All published articles of this journal are available on ScienceDirect.
Morphological, Biochemical, and Molecular Analysis of Origanum vulgare L.
Abstract
Background:
The variation on morphological, biochemical, and genetic characters is very important in germplasm management and conservation strategies.
Objective:
To determine the diversity of 23 accessions from Origanum vulgare L. and a commercial cultivar on the basis of agronomical, biochemical and genetic features.
Methods:
Different characters related to vegetative growth and essential oil production were studied while the genetic relationships between the individuals were evaluated with the use of Amplified Fragment Length Polymorphism.
Results:
Despite the accessions exhibited cymyl- and acyclic-compounds, all the essential oil chemotypes according to the prevalence of essential oil fractions were phenotypically varied. A considerable amount of biomass with maximum values in plant height was achieved by thymol/γ-terpinene chemotype and carvacrol chemotype making them of particular interest for the production of high-quality plant material and further for the mechanical harvest. The AFLP analysis, performed using 10 primer combinations, to obtain a dendrogram of genetic similarity, revealed a genetic variability that could be useful for the selection of the proper genetic groups in future breeding programs.
Conclusion:
We identified two chemotypes thymol/γ-terpinene and carvacrol with their suitability for the production of biomass and essential oil and for the mechanical harvest. The results of the molecular characterization of the species may support and contribute to breeding programmes for agronomic and biochemical traits.
1. INTRODUCTION
The genus Origanum belongs to Lamiaceae family, which grows wildly in Mediterranean areas and northern Africa [1]. It includes various species, subspecies, varieties and hybrids that can be distinguished individually with a high variation in morphological and chemical features [2]. According to the Ietswaart’s classification [3], no less than 10 sections, consisting of 49 taxa, had to be recognized on the basis of geographical distribution. Origanum is a monospecific section which includes six subspecies of Origanum vulgare: Gracile (Kock) Ietswaart, glandulosum (Desfontaines) Ietswaart, hirtum (Link) Ietswaart, vulgare L., virens (Hoffmannsegg et Link) Ietswaart, and viride (Boissier) Hayek. They were recognized based on the indumentum, number of sessile glands on leaves, bracts and calyces and the size and colour of bracts and flowers. The southernmost range of O. vulgare in Europe comprises three subspecies: O. vulgare L. subsp. Glandulosum (Desfontaines) Ietswaart, O. vulgare L. subsp. hirtum (Link) Ietswaart, and O. vulgare L. subsp. gracile (Koch) Ietswaart, found to be rich in the EOs while the northern part that includes the other two, O. vulgare L. subsp. virens (Hoffmannsegg et Link) Ietswaart, O. vulgare L. subsp. vulgare L. and O. vulgare L. subsp. viride (Boissier) Hayek, pointed out to be poor in EOs [4].
Oregano plants are known to possess a special composition of Essential Oil (EO) which is of great interest for pharmaceutical and industrial market [5, 6]. Furthermore, it was grouped into different chemotypes: Acyclic, cymyl, bicyclic, and/or bornane type compounds depending on the predominance of particular components as (a) linalool, linalyl acetate, β-ocimene and myrcene; (b) p-cymene, γ-terpinene, p-cymene-8-ol, thymol methyl ether, carvacrol methyl ether, thymoquinone, thymol and carvacrol; (c) sabinene, trans-/cis-sabinene hydrate and their acetate; and (d) camphor, borneol, bornyl acetate, respectively [1, 4, 7-10].
In this context, the active compounds of EO are not only responsible for the aroma and flavor of oregano, making O. vulgare the most popular spice for food production, but are also emerged as effective source to provide a vast range of biological activities with antioxidant [11], antifungal [12], antibacterial [13], anticancer [14], insecticidal [15], herbicidal [16] and nematicidal [17] properties.
The quantitative and qualitative profile of EO is principally influenced by the genotype, the environmental factors, the growing conditions, and the geographical locations [18-21] leading to a noticeable variation between populations and accessions of O. vulgare and within the genus Origanum by itself [22-24].
For better management of oregano genetic resources, extensive efforts to improve cultivars and restore endangered varieties [25] with a higher content of bioactive compounds are urgently needed. Moreover, extending the research about the morphological and chemical diversity of oregano populations is very important. Furthermore, oriented exploitation of its germplasm in Italy could enable plant breeders of selecting the interesting parental sources for breeding programs [26].
The safeguard of genetic resources implies not just the collection and the storage of material but also the evaluation and the characterization through morphological, biochemical and molecular analysis. All this should be done to prevent the conservation of redundant genetic materials that do not induce important variation and that contribute to increasing the biodiversity management cost [27, 28]. Moreover, the assessment of genetic diversity within collections becomes significant to identify genotypes of agronomic interest, useful in crop-improvement programs.
The molecular analysis, able to evaluate DNA polymorphism to univocally characterize and estimate the genetic distances among plant materials, was performed using AFLP (Amplified Fragment Length Polymorphism) markers on DNA isolated from leaves. The choice of AFLPs is due to the potential capability of this technique to detect a large level of polymorphism, particular when combined with the fluorescence detection system. AFLP is used for different applications, such as analysis of population polymorphism, phylogenetic relationship, genetic diversity assessment, identification of loci linked to economically valuable traits, genetic maps design and varietal fingerprinting [29-32]. This method also can be used by breeders to preliminary assess the initial genetic material to plan the strategy of crosses, to identify the best combinations of genotypes, and for general selection [33, 34].
The aim of the current study was to determine the morphological and the phytochemical variability within different O. vulgare accessions collected from various geographical areas of Southern Italy and to show the usefulness of AFLP technique in the assessment and genetic characterisation, making possible the identification of promising material to use in future breeding programs besides evaluating the chemical essential oil composition in order to identify genotypes with higher content in carvacrol, thymol and γ-terpinene.
2. MATERIALS AND METHODS
2.1. Plant Material and Experimental Field
A total of 24 accessions of O. vulgare were investigated, 23 wild populations collected, according to Good Agricultural Practices (GAP) [35], from different locations of Southern Italy (Apulia, Basilicata and Calabria) along with one commercial cultivar “Sais” (S.A.I.S., S.p.a., Cesena, Italy), belonging to the genus Origanum. Additional information related to the coordinates (i.e latitude, longitude) plus the altitude were provided in Table 1. All oregano plants were identified and then cuttings of each accession were grown at Enrico Pantanelli farm of the University of Bari Aldo Moro (Policoro, Italy). The soil of this experimental farm is loamy (sand 398 g/kg, silt 374 g/kg clay 228 g/kg). The chemical characteristics of soil as described by Pontonio et al. [36]: pH 7.7; 2.3% organic matter, 1.7 g/kg total N (Kjeldahl method) and 27.6 mg/kg available P2O5 (Olsen method). The experimental design was a randomized complete block with 3 replications following GAP for medicinal plants [35]. The planting distance was 0.75 m between rows and 0.35 m within rows.
2.2. Agronomical Evaluation/Collection Data
The plants were collected during the full flowering between the end of June and the middle of July. Different characters related to vegetative growth and leaves production including days’ number of early flowering, plant height, biomass, and leaf area, were measured with 15 individuals per each population.
2.3. Extraction of Essential Oils
The aerial parts of all the investigated accessions were dried (air drying oven, 35 °C) and then a fraction of each (20 g) was submitted, according to European Pharmacopoeia [37], to hydro-distillation (Clevenger apparatus, 4h). The obtained EOs were dried over anhydrous sodium sulfate and stored at 4 °C, in amber vials, until further analysis. The oil content was calculated as v/w on the basis of the dry matter of the initial material.
| Accession | Collection Site | Province |
Altitude of Collection Site (m a.s.l.) |
Latitude | Longitude | Sum of | |||
|---|---|---|---|---|---|---|---|---|---|
| N | E | Acy1 | Cym | Sab | Ses | ||||
| Or1 | Bitonto (Ba) | Bari | 118 | 41°06′30.00″ | 16°41′30.00″ | 3.29 | 86.06 | 0.79 | 6.12 |
| Or2 | Carovigno (Br) | Brindisi | 161 | 40°42′26.00″ | 17°39′34.00″ | 21.20 | 54.40 | 7.82 | 9.01 |
| Or3 | Castellaneta (Ta) | Taranto | 235 | 40°38′00.00″ | 16°56′00.00″ | 9.25 | 69.65 | 5.08 | 13.06 |
| Or4 | Policoro (Mt) | Matera | 25 | 40°12′00.00″ | 16°40′00.00″ | 1.66 | 87.01 | 1.13 | 5.91 |
| Or5 | Murgia A (Ba) | Bari | 397 | 40°49′45.28″ | 16°24′59.07″ | 6.73 | 71.33 | 0.84 | 16.58 |
| Or6 | Casarano (Le) | Lecce | 107 | 40°01′00.00″ | 18°10′00.00″ | 11.37 | 62.14 | 0.00 | 23.85 |
| Or7 | Locorotondo (Ba) | Bari | 410 | 40°45′00.00″ | 17°19′00.00″ | 11.47 | 60.87 | 0.00 | 25.25 |
| Or8 | Valenzano (Ba) | Bari | 85 | 41°03′00.00″ | 16°53′00.00″ | 5.50 | 76.76 | 0.00 | 13.84 |
| Or9 | Varano (Fg) | Foggia | 165 | 41°50′00.00″ | 15°46′00.00″ | 2.18 | 82.53 | 0.43 | 10.61 |
| Or10 | Modugno A (Ba) | Bari | 43 | 41°05'07.80” | 16°48'51.60” | 8.94 | 73.30 | 0.95 | 12.78 |
| Or11 | Modugno B (Ba) | Bari | 80 | 41°04'45.30” | 16°47'11.50” | 6.99 | 78.32 | 0.40 | 7.81 |
| Or12 | Modugno C (Ba) | Bari | 101 | 41°04'22.70” | 16°45'36.90” | 22.90 | 33.87 | 0.00 | 36.76 |
| Or13 | Pollino A (Pz) | Potenza | 735 | 39°57'18.20” | 16°06'33.60” | 73.62 | 0.00 | 5.47 | 19.04 |
| Or14 | Pollino D (Pz) | Potenza | 951 | 39°56'54.50” | 16°07'34.20” | 80.77 | 0.00 | 0.00 | 15.04 |
| Or15 | Pollino G (Pz) | Potenza | 843 | 39°53'11.30” | 16°09'13.20” | 72.66 | 1.80 | 0.00 | 23.68 |
| Or16 | Spinazzola (Ba) | Bari | 435 | 40°58′00.00″ | 16°05′00.00″ | 10.05 | 64.07 | 0.00 | 19.50 |
| Or17 | Murgia B (Ba) | Bari | 394 | 40°37'49.30” | 16°40'11.10” | 19.15 | 64.57 | 0.00 | 13.03 |
| Or18 | Murgia C (Ba) | Bari | 362 | 40°38′48.33″ | 17°06′35.20″ | 18.09 | 54.01 | 0.00 | 23.68 |
| Or19 | Putignano (Ba) | Bari | 372 | 40°50′57.22″ | 17°07′21.16″ | 5.16 | 72.02 | 0.00 | 18.23 |
| Or20 | Ostuni (Br) | Brindisi | 229 | 40°43′56.00″ | 17°34′40.00″ | 8.20 | 70.74 | 0.00 | 16.49 |
| Or21 | Ceglie Messapica (Br) | Brindisi | 302 | 40°39′00.00″ | 17°31′00.00″ | 15.47 | 67.65 | 0.00 | 13.22 |
| Or22 | Vibo Valentia (Vv) | Vibo Valentia | 421 | 38°40'51.90” | 16°06'02.40” | 6.43 | 70.27 | 0.00 | 21.06 |
| Or23 | Catanzaro (Cz) | Catanzaro | 320 | 38°54′ 36.00″ | 16°35′ 15.00″ | 6.35 | 75.35 | 0.00 | 15.67 |
| Or24 | Sais | Commercial cultivar | - | - | 9.73 | 0.00 | 36.70 | 51.02 | |
2.4. Analysis of Essential Oils Using Gas Chromatography-Mass Spectrometry
The GC-MS analysis was done by Agilent 6890N gas chromatograph coupled to an Agilent mass spectrometer 5973N (Agilent Technologies, Cernusco sul Naviglio, MI, Italy). The separation of the volatile compounds was carried out on a capillary column HP-5MS (30m ×0.25mm × 0.25μm film thicknesses). The carrier gas was Helium (flow rate, 1.1 ml min-1), the temperature of the injector and transfer line were set to 250 and 300, respectively. The initial column temperature was 60 °C, then ramped to 110 °C at the rate of 2 °C min-1 and again to 220 °C at 10 °C min-1. 1.0 μl of the sample was submitted to GC–MS using the split mode (split ratio 1:50). A mixture of aliphatic hydrocarbons (C8−C30; Sigma, IT-Milan) in n-hexane was directly injected into the GC under the same analytical conditions in order to calculate the Retention Indices (RIs) of peaks in the chromatogram. All the mass spectra were acquired using the Electron-Impact (EI) mode with an ionization voltage of 70 eV [38].
2.5. Identification of EO Composition
The constituents of the essential oils were identified based on the retention index, mass spectra obtained from Wiley [39], NIST [40] and Adams [41] libraries and those reported in the literature. The content of each component corresponded to the relative percentage of the total peak area without the use of correction factors [42].
2.6. DNA Extraction and AFLP Analysis
DNA was extracted from tissue leaf by mean of a commercial kit (GenEluteTMPlant Genomic DNA Kit -Sigma-Aldrich Chemie, Steinheim, Germany). Quality and quantity of genomic DNA were evaluated both on 0.8% agarose gel electrophoresis, comparing band intensity of DNA samples to Lambda DNA standard and using the Nano Drop 2000 UV-Vis Spectrophotometer (ThermoScientific, Waltham, Massachusetts, US). AFLP analyses were performed as reported by Vos et al. [43]. DNA (50 ng) was digested with two restriction enzymes: the six-cutter EcoRI and the four-cutter MseI. Restriction fragments size were assessed on 1.5% agarose gel. Adapters were ligated to fragments and ligation products were diluted 1:10 in TE (Tris-HCl 100mM, pH 8.0, EDTA 0.01 mM). Pre-selective and selective PCR was performed as reported in the AFLP-TM Plant Mapping Kit Manual (Applied Biosystems Corp., Norwalk, Connecticut, U.S.) In Tables 2 and 3 sequences of adapters and primers are reported. Labeled fragments obtained by using FAM, NED and JOE EcoRI primers were analyzed on an ABI PRISM 310 Automated DNA Sequencer (Applied Biosystems Corp., Norwalk, Connecticut, U.S.).
| MseI Adapter | 5’-GACGATGAGTCCTGAG-3’ 3’-TACTCAGGACTCAT-5’ |
| EcoRI Adapter | 5’-CTCGTAGACTGCGTACC-3’ 3’-CTGACGCATGGTTAA-5’ |
| MseI Primer | 5’- GATGAGTCCTGAGTA 3’ |
| EcoRI Primer | 5’-GACTGCGTACCAATTC 3’ |
| Primer Combinations | Total Bands (n.) | Polymorphic Bands (n.) | Polymorphism (%) |
|---|---|---|---|
| E-ACA/M-CTG | 244 | 87 | 35.6 |
| E-ACA/M-CTT | 250 | 112 | 44.8 |
| E-AAC/M-CTC | 332 | 132 | 39.7 |
| E-AAC/M-CAC | 357 | 128 | 35.8 |
| E-ACG/M-CAT | 225 | 68 | 30.2 |
| E-ACG/M-CTA | 361 | 115 | 31.8 |
| E-ACG/M-CTC | 382 | 129 | 33.7 |
| E-ACG/M-CAG | 325 | 107 | 32.9 |
| E-ACA/M-CTC | 282 | 94 | 33.3 |
| E-ACA/M-CAG | 557 | 225 | 40.4 |
| 3315 | 1179 | 35.8 |
2.7. Statistical Analysis
The results obtained from growth and yield characteristics are expressed as mean±standard deviation while the EO components data are presented as mean. All scored bands in AFLP analysis were processed using the Genotyper software (A.B. Corp., Norwalk, Connecticut, U.S.) obtaining a binary matrix based on the presence (1) or absence (0) of each polymorphic DNA fragment detected on the 24 genotypes. The software GenAlex 6.5 [44] was used to calculate allele frequencies, Nei distance matrix and to perform PCo analysis (PCoA). A dendrogram of genetic distance was constructed using the Unweigth Pair Group Method with Aritmetic averages (UPGMA) implemented in the Mega software 6.0 [45].
3. RESULTS
The research led to identifying 23 accessions belonging to different sites located in Apulia, Basilicata and Calabria regions- Southern Italy, from which 23 accessions of oregano were collected. All populations were classified as Origanum vulgare L. For a clear presentation of the data, the collected plants were grouped into cymyl-compounds and acyclic-compounds according to the metabolic pathway EO fractions (Table 1). OR13, OR14, OR15 were regarded as acyclic pathway (sum of linalool, linalyl acetate, β-ocimene and myrcene peak area percentage) while all the other populations were exhibited cymyl pathway (sum of p-cymene, γ-terpinene, thymol methyl ether, carvacrol methyl ether, thymol and carvacrol peak area percentage) except OR24 was classified into mixed chemotype including the sabinyl pathway (sum of sabinene, trans-sabinene hydrate and cis-sabinene hydrate peak area percentage) with a presence of sesquiterpenes compounds.
3.1. Growth and Yield Characteristics
Mean and standard deviation values for the studied measured characters are reported in Table 4. Eight chemotypes according to the prevalence of EOs compounds were phenotypically diverse. Plant height was the lowest (52.01cm) in carvacrol methyl ether/γ-terpinene chemotype while it was the highest in oregano populations belonging to carvacrol (76.70cm) and thymol/γ-terpinene (75.20cm) chemotypes. Carvacrol chemotype also reported the maximum values in early flowering days (151.33) compared to 139.67 in linalyl acetate chemotype. The leaf area ranged from 1.01cm2 leaf-1 (chemotype of carvacrol methyl ether/γ-terpinene) to 2.74 cm2 leaf-1 (carvacrol chemotype). In addition, the biomass (g plant-1) harvested from carvacrol chemotype (248.80) was comparable with the other chemotypes (113.33-185.33) but less than thymol/γ-terpinene chemotype (257.83).
3.2. Content of Essential Oil Among Oregano Populations
It was evident that the presence of variation in EO content among all the oregano populations (Table 2), carvacrol group was the richest (4.02) compared to γ-terpinene group. It seems that the potential yield of accessions contained γ-terpinene in combination with carvacrol methyl ether was decreased (1.01%) to be less than of OR2, OR5, OR7, OR8, OR12, OR18 (1.28%) where γ-terpinene was the main component. However, the populations of linalyl acetate, thymol, thymol/ γ-terpinene, thymol/γ-terpinene/p-cymene and sabinyl-compounds+sesquiterpenes (β-caryophyllene) characterized by a high essential oil content (2%) than the other two populations γ-terpinene and carvacrol methyl ether/γ-terpinene (~1%).
3.3. Chemical Analysis of Essential Oil
A total of 43 compounds were identified in the 23 studied populations and single cultivar which further classified as the following: thirteen are monoterpenes hydrocarbons (11.14- 61.11%), twelve of oxygenated monoterpenes (1.48-68.89%), two of phenolic monoterpenes (4.30-70.24%), twelve of sesquiterpene hydrocarbons (5.69-44.93%), three oxygenated sesquiterpene (0.22-6.83%) and one is uncategorized compound. Only the main EO constituents obtained from 24 oregano accessions are presented (Table 5) where is the variation in the chemical composition is quite remarkable (84.94-94.69%). In OR1, OR4 and OR9 the main constituent found to be carvacrol with a total of 72.20%, 70.03 and 69.03%, respectively (carvacrol chemotype) while the other phenolic monoterpene (thymol) was the major compound in OR10, OR11, OR16, OR17, OR19 having a higher percentage from 32.85% to 52.03% (thymol chemotype). Linalyl acetate was the first main oxygenated monoterpenes with 59.36, 59.59 and 66.30% respectively in OR13, OR14 and OR15 (linalyl acetate chemotype) while carvacrol methyl ether was interestingly the highest in OR22 population (25.65%). Among the monoterpenes hydrocarbons, γ-terpinene appeared to be the most dominant in the essential oils from twelve accessions tested, ranging from 18.08% to 34.95% followed by p-cymene (18.67%) which detected only in OR23 or sabinene (36.70%) in OR24.
3.4. AFLP Fingerprinting and Genetic Similarity
The 10 AFLP primer combinations yielded a total of 3315 scorable fragments, of which 1179 were polymorphic. The number of polymorphic bands generated by each AFLP primer combination (Table 3) varied from 225 (E-ACG/M-CAT) to 557 (E-ACA/M-CAG). As shown in Table 3, the 10 primer combinations detected 35.8% of polymorphism.
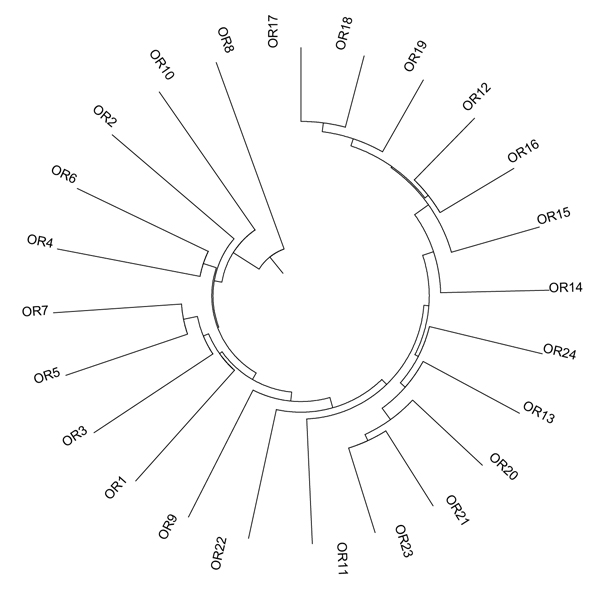
Diversity among genotypes was assessed by the construction of a UPGMA dendrogram (Fig. 1), showing a wide variability among genotypes. Three main clusters are valuable: the first grouped five genotypes: from OR8 to OR4; the second seven genotypes from OR7 to OR11; the third genotypes from OR23 and OR17.
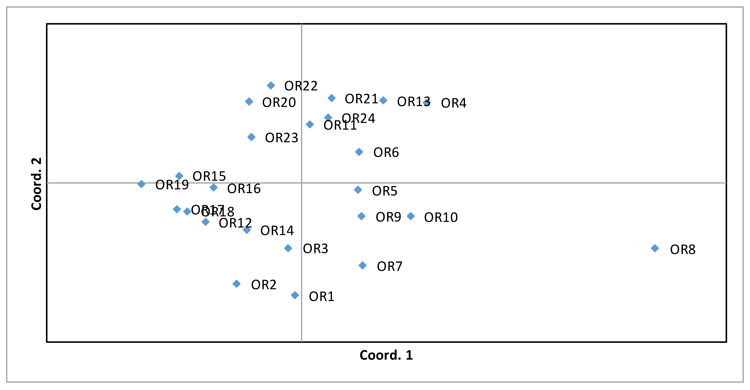
PCoA analysis was also performed (Fig. 2), supporting the evidence of wide variability among genotypes, in which only OR8 genotype is not grouped with the remaining ones.
| Chemotype | Accession | Early Flowering | Plant Height (cm) |
Biomass (g plant-1) |
Leaf Area (cm2 leaf-1) |
Essential Oil Content (% v/w) |
|---|---|---|---|---|---|---|
| Linalyl acetate | OR13,OR14,OR15 | 139.67±5.77 | 59.63±20.39 | 113.33±56.15 | 2.27±0.33 | 2.02±0.12 |
| Carvacrol | OR1, OR4, OR9 | 151.33±4.04 | 76.70±4.01 | 248.80±83.87 | 2.74±0.69 | 4.02±1.25 |
| Thymol | OR10,OR11,OR16,OR17,OR19 | 144.20±2.68 | 68.40±6.26 | 144.12±81.85 | 2.55±0.92 | 1.91±1.23 |
| Thymol/γ-terpinene | OR3,OR6,OR20,OR21 | 147.50±3.00 | 75.20±9.05 | 257.83±35.68 | 2.69±0.41 | 2.05±0.56 |
| γ-terpinene | OR2,OR5,OR7,OR8,OR12,OR18 | 145.33±6.50 | 69.22±16.07 | 185.33±91.43 | 2.61±0.85 | 1.28±0.53 |
| Carvacrol methyl ether/γ-terpinene | OR22 | 149.50±0.71 | 52.01±1.00 | 81.80±1.84 | 1.01±0.01 | 1.01±0.01 |
| Thymol/γ-terpinene/p-cymene | OR23 | 150.00±0.00 | 62.26±2.74 | 134.72±6.39 | 1.05±0.06 | 2.16±0.20 |
| Sabinyl-compounds+sesquiterpenes (β-caryophyllene) | OR24 | 145.43±0.57 | 69.46±0.92 | 178.01±4.10 | 2.30±0.21 | 2.25±0.30 |
| Accession | RI1 | OR1 | OR2 | OR3 | OR4 | OR5 | OR6 | OR7 | OR8 | OR9 | OR10 | OR11 | OR12 | OR13 | OR14 | OR15 | OR16 | OR17 | OR18 | OR19 | OR20 | OR21 | OR22 | OR23 | OR24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | |||||||||||||||||||||||||
| Thymol methyl ether | 1235 | -2 | 1.6 | 5.1 | - | 11.64 | 5.92 | 5.43 | 3.87 | 0.78 | 1.76 | 0.56 | - | - | - | - | 7.06 | 1.95 | 1.21 | 5.85 | 3.14 | 8.75 | - | 11.52 | - |
| Linalyl acetate | 1257 | - | 8.52 | - | - | - | - | - | - | 0.06 | - | - | - | 66.303 | 59.36 | 59.59 | - | - | - | - | - | - | - | - | - |
| Germacrene D-4-ol | 1574 | - | - | 4.00 | - | 1.46 | 5.45 | 6.62 | 2.23 | - | 2.64 | 0.26 | 2.64 | 6.83 | 2.23 | 5.28 | 1.89 | 2.43 | 1.36 | 2.7 | 3.02 | 0.74 | - | - | 4.99 |
| Octen -3-yl-acetate | 1110 | - | 0.81 | - | - | - | - | - | - | - | - | - | - | 0.93 | 3.18 | 1.24 | - | - | - | - | - | - | - | - | - |
| Carvacrol | 1298 | 72.2 | - | 5.66 | 70.03 | 0.24 | 1.64 | 0.55 | 0.25 | 69.03 | 4.71 | 1.31 | - | - | - | - | 1.71 | 2.39 | 1.34 | 1.76 | - | - | 15.95 | 2.29 | - |
| γ-terpinene | 1062 | 9.79 | 30.19 | 24.41 | 11.87 | 26.89 | 25.68 | 25.95 | 34.95 | 10.13 | 13.99 | 16.05 | 26.93 | - | - | 1.80 | 9.27 | 17.39 | 29.06 | 21.41 | 28.46 | 25.75 | 23.4 | 18.08 | - |
| p-cymene | 1022 | 3.5 | 7.55 | 4.52 | 4.91 | 6.90 | 4.26 | 4.22 | 4.50 | 2.38 | 7.98 | 5.89 | 2.64 | - | - | - | 4.13 | 3.79 | 3.21 | 7.00 | 4.31 | 2.78 | 5.28 | 18.67 | - |
| β-caryophyllene | 1418 | 3.08 | 3.42 | 2.82 | 3.23 | 4.23 | 8.66 | 8.93 | 3.43 | 2.88 | 3.40 | 0.92 | 10.85 | 4.06 | 3.65 | 5.22 | 6.20 | 1.69 | 2.72 | 5.4 | 3.94 | 3.45 | 8.34 | 4.93 | 23.81 |
| Cis-β-ocimene | 1040 | 1.62 | 4.26 | 3.27 | - | 3.96 | 5.69 | 5.42 | 2.86 | 0.16 | 6.56 | 4.66 | 14.48 | 2.16 | 15.84 | 8.66 | 7.84 | 4.13 | 6.74 | 2.53 | 5.57 | 5.17 | 3.87 | 3.19 | 6.94 |
| Germacrene A | 1503 | 1.03 | 2.19 | 1.64 | 0.22 | 0.58 | 1.69 | 1.77 | 1.36 | 0.58 | 1.61 | 0.93 | 5.59 | 0.67 | 1.84 | 3.20 | 2.08 | 1.28 | 5.47 | 0.86 | 1.55 | 0.84 | 2.36 | 1.22 | 2.63 |
| Thymol | 1290 | 0.3 | 13.49 | 27.74 | 0.21 | 22.77 | 22.18 | 22.26 | 30.57 | 0.21 | 44.85 | 52.03 | 4.30 | - | - | - | 38.03 | 36.3 | 17.72 | 32.85 | 32.01 | 27.67 | - | 21.82 | - |
| Carvacrol methyl ether | 1244 | 0.27 | 1.57 | 2.23 | - | 2.89 | 2.45 | 2.45 | 2.61 | - | - | 2.48 | - | - | - | - | 3.87 | 2.74 | 1.48 | 3.15 | 2.83 | 2.69 | 25.65 | 2.97 | - |
| Germacrene D | 1480 | 0.25 | 3.15 | 1.98 | 0.41 | 7.26 | 6.85 | 6.68 | 5.25 | 0.86 | 2.49 | 3.73 | 14.27 | 5.86 | 6.80 | 4.91 | 6.10 | 6.39 | 11.45 | 6.09 | 4.56 | 6.1 | 8.87 | 3.58 | 13.81 |
| Sabinene | 976 | 0.11 | 7.82 | 5.08 | 0.11 | - | - | - | - | 0.11 | - | - | 5.47 | - | - | - | - | - | - | - | - | - | - | 36.7 | |
| Linalool | 1098 | 0.07 | 2.45 | 1.81 | 0.05 | 0.93 | 3.06 | 3.37 | 1.05 | 0.46 | - | 0.24 | 3.24 | 1.65 | 1.33 | 2.16 | - | 12.74 | 8.54 | 0.91 | - | 7.97 | 0.98 | 1.69 | - |
4. DISCUSSION
4.1. Agronomic Characteristics
The focus of the current study was to investigate the variability among different wild-growing O. vulgare accessions. Populations belonging to thymol/γ-terpinene chemotype (4 accessions) and carvacrol chemotype (3 accessions) have produced a considerable amount of the biomass, making them of particular interest for biomass-production purposes and further for the production of essential oils [46]. In addition to their individuals reached maximum values in plant height which is of great importance in medicinal plant breeding for the mechanical harvest [47]. For most of the evaluated characters, a noticeable heterogeneity between populations were observed in the present study. Our results are in agreement with the available literature, where a substantial variable in plant height and dry mass have been underlined in a germplasm collection of Origanum vulgare L. from Europe [19] as well as wild Tunisian oregano [48]. Also, the Hungarian Origanum vulgare populations showed a high degree of variability in response to altitude, soil type and humus content [49]. This indicates the importance of location features on oregano growth which can further illustrate the high variation in plant performance under different environments [50].
4.2. EO Content
The essential oil of O. vulgare samples has been analysed and its chemotypes were identified [51]. Essential oil contents from leaves and flowers, ranged between carvacrol chemotype, as the most productive group to reach the lowest content in carvacrol methyl ether/γ-terpinene chemotype. The correlation between the oil yield and carvacrol content was strongly reported in populations featured by a very high amount of carvacrol and EO content as well [52, 53]. Therefore, best EO content in OR1, OR4, OR9 (carvacrol chemotype) accompanied by the maximum presence of carvacrol up to 72.20% [54]. The other chemotypes of O. vulgare accessions were poor in EO content (<2%) as OR22, OR2, OR5, OR7, OR8, OR12 and OR18 in which the main constituents were γ-terpinene, carvacrol methyl ether/γ-terpinene [55]. In detail, the monoterpene fraction of the above essential oil-poor accessions comprised a higher cymyl-compounds (γ-terpinene, carvacrol methyl ether) whereas the acyclic compounds were minimized or even deactivated. Such an observation led to exceptional chemotypes with ‘low quality’ plant material poor in phenolic monoterpene which is less important for commercial purposes [9]. This confirms the difficulties to ensure large scale production, from a wild collection of native populations, with the better quality required by consumers. Generally, the diversity in the accumulation of EO constituents and commonly the carvacrol content is one of the most important breeding goals, which in turn has led to improve cultivars of great importance in oregano species [51].
4.3. EO Composition and Main Chemotypes
Over forty mono- and sesquiterpenes have been reported from the complex and highly variable essential oil of Origanum vulgare plants. Almost 60% of these compounds were monoterpenes from acyclic or cymyl-pathway, including carvacrol, thymol, γ-terpinene, and p-cymene while sabinyl-compounds with the presence of sesquiterpenes (mainly β-caryophyllene and Germacrene D) was only reported in few cases. Comparing the current data with results from other studies demonstrated considerable variability in the chemical profile and the identified chemotypes of oregano EO either for the single compounds or even for the biosynthetic pathway. Most of the papers described the essential oil composition of O. vulgare from different geographic areas. Sicilian oregano was found to be rich in thymol (phenolic monoterpenes) with a variable percentage of γ-terpinene, p-cymene, and carvacrol [56] or thymol (phenolic monoterpenes) and γ-terpinene (monoterpenes hydrocarbons) followed by p-cymene, carvacrol and thymol methyl ethers [57]. Recently, native populations of this species from Calabria region showed the predominate of carvacrol, thymol and γ-terpinene as well as linalyl acetate (oxygenated monoterpenes) which it resulted from the active acyclic pathway [21]. More chemotypes have been determined also in Calabria on the basis of the phenolic content, i.e., thymol, carvacrol, thymol/carvacrol, and carvacrol/thymol chemotypes [58]. It was also underlined the differentiation in EOs composition based on the metabolic pathway, sesquiterpenes rich type and partly sabinyl compounds were the characteristic of O. vulgare individuals native to northern Italy [9]. In an earlier investigation on oregano EOs from Liguria and Emilia regions, various groups have been identified: the first was rich in carvacrol/thymol content, the second had a high prevalence of linalool with sesquiterpenes and lastly, the third was characterized by the abundance of sesquiterpenes [7]. Likewise, the relationship between chemical composition and biotypes and/or chemotypes was stated in three chemotypes from Campania region: one rich in carvacrol/thymol while the other two were described for the first time as thymol/α-terpineol and linalyl acetate/linalool [59]. Despite the fact that numerous data reported wide differences in monoterpenes accumulation depending on geographical areas, the findings obtained from the present research possibly correlated to the different growing conditions, in which the analyzed plants were collected [58]. Another point probably indicates the dissimilarity of EO chemotypes in specific area, is that the pollination in O. vulgare plants is mostly cross [60], which can cause a wide variation in the levels of carvacrol, thymol, p-cymene, and γ-terpinene in the essential oils as a result of sexual polymorphism or genetic mechanism [61].
4.4. AFLP Fingerprinting and Genetic Similarity
AFLP analysis is a powerful tool to analyze germplasm investigating the whole genome. The choice of this class of markers was also due to a low efficiency showed by using RAPD markers on the 24 oregano genotypes (data not shown), revealing not reproducible data and, for some genotypes, lack of amplification patterns.
Furthermore, polymorphisms were detected using a fluorescence system, allowing to improve the efficiency of the classical AFLP procedure, based on radioactive detection of bands after electrophoresis on polyacrylamide gels. In fact, AFLP analysis was also performed on oregano by other Authors, detecting a lower number of total and polymorphic bands [1, 19, 62].
Polymorphism level detected on the 24 oregano genotypes was lower in respect to data reported by Azizi et al. [1], who analyzed a wide germplasm collection from different Countries.
CONCLUSION
The wild O. vulgare accessions collected from different locations of Southern Italy have been evaluated in terms of morphological, biochemical and molecular properties. Considerable variability in the EO profile among the oregano populations was underlined, leading to identify 4 accessions belong to thymol/γ-terpinene chemotype and other 3 accessions from carvacrol chemotype. Such accessions are of great interest for biomass and EO production besides the suitability for the mechanical harvest since their individuals reached maximum values in plant height.
Molecular characterization, performed by means of AFLPs, showed a wide genetic variability within oregano germplasm. Due to the scant information about the molecular characterization of the species results obtained in the present work could support and contribute to breeding programmes for agronomic and biochemical traits.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
RESEARCH INVOLVING PLANTS
The reported experiments were in accordance with the United Nations (UN, 1992) convention on biological diversity.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
All individuals listed as authors were contributed substantially to the design, performance, analysis, and reporting of the work.


